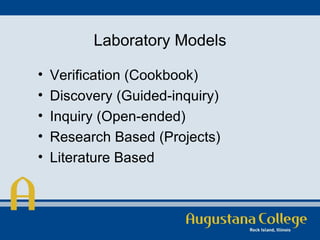
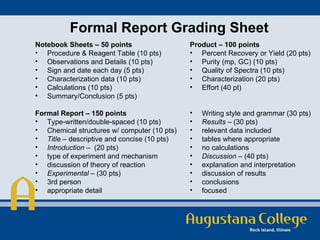
The document discusses a new model for organic chemistry laboratory education, emphasizing the need for improved student engagement and the development of critical thinking skills. It identifies issues within traditional labs, such as lack of verification and purpose, and proposes guided inquiry and research-based learning methods to enhance student independence and communication skills. The model includes detailed experimental procedures and evaluation criteria aimed at bridging gaps between lecture and lab performances.


















![In Closing Copies of the Lab Syllabi Thanks to Tredway Library, Augustana College Chemistry department colleagues Augustana students Contact us at: [email_address] [email_address]](https://image.slidesharecdn.com/bccelab2008-1231262434787587-1/85/Bcce-Lab2008-22-320.jpg)