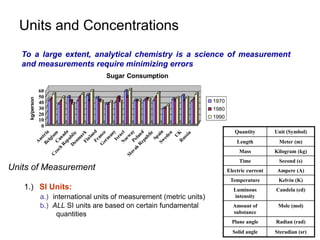
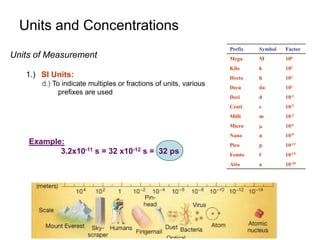
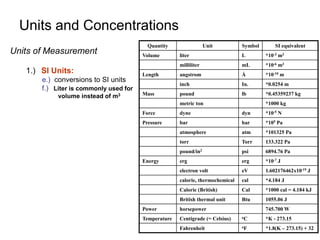
This document provides information about an introductory quantitative analysis chemistry course. It outlines the course details including instructors, meeting times, exams, grading breakdown, textbook, and lab schedule. It also describes the course expectations for labs, lab notebooks, and homework problems. Key concepts covered in lectures and experimental techniques are briefly introduced.