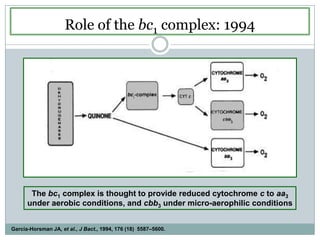
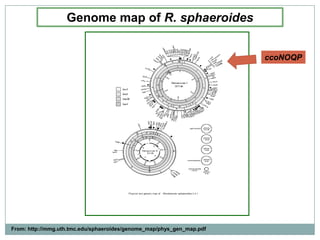
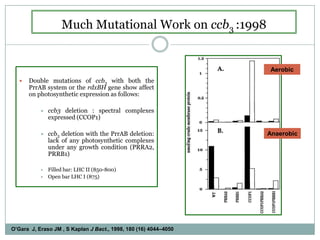
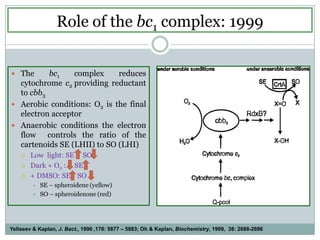
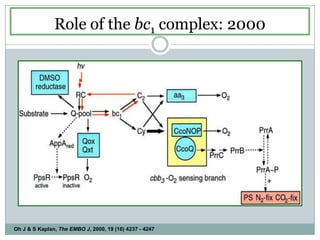
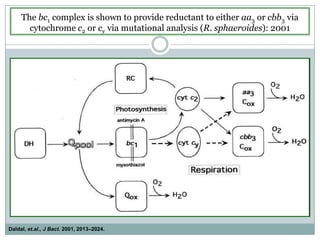
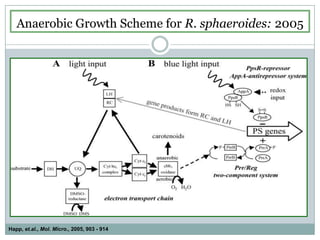
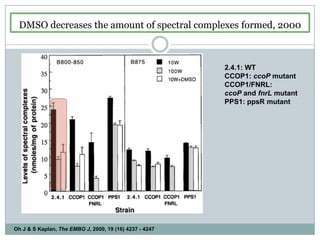
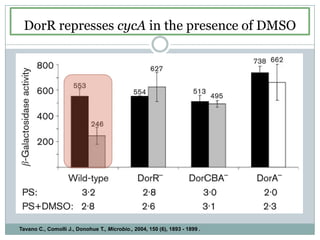
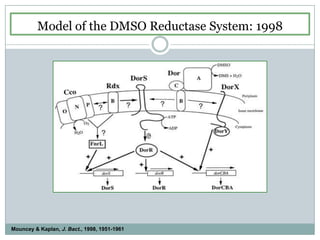
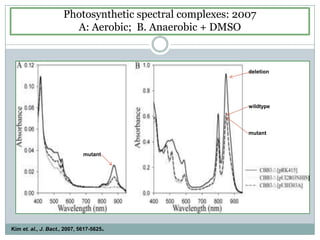
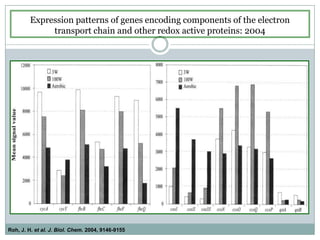
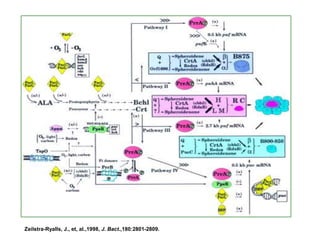
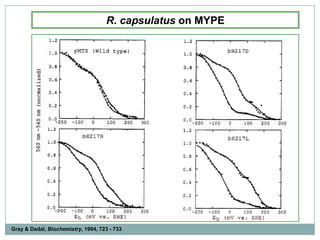
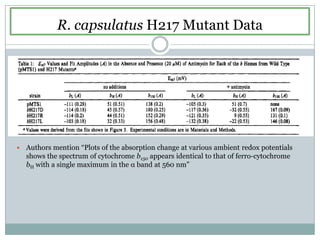
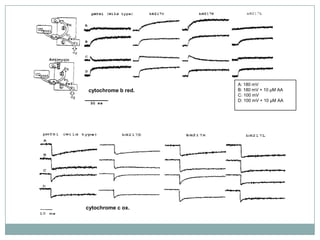
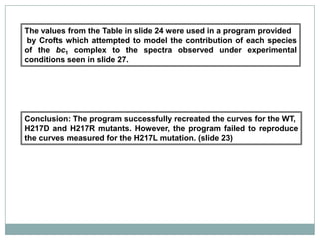
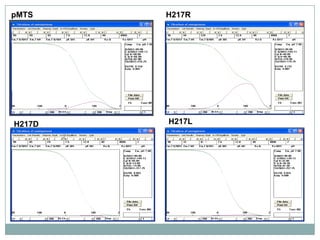
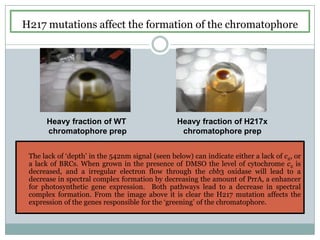
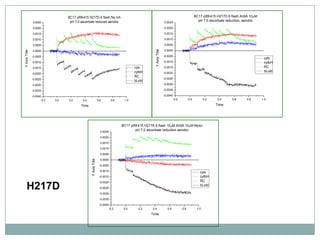
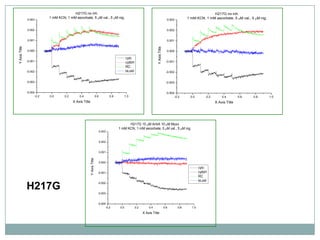
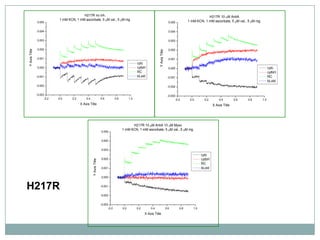
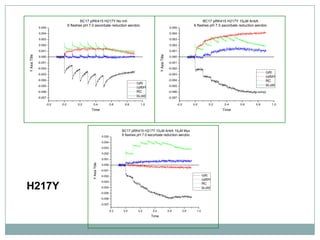
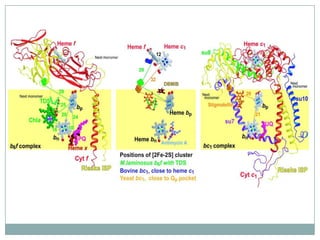
The bc1 complex provides reduced cytochrome c to either the aa3 or cbb3 oxidases depending on oxygen conditions in R. sphaeroides. Strain BC-17 cannot photosynthesize due to lack of bc1 complex reducing cbb3. Some H217 mutations in the QI site can photosynthesize with DMSO but revert or are lethal without it. Future work should introduce H217 mutations into a DorR-/PpsR- background to uncouple effects on bc1 from changes to photosystem expression levels.