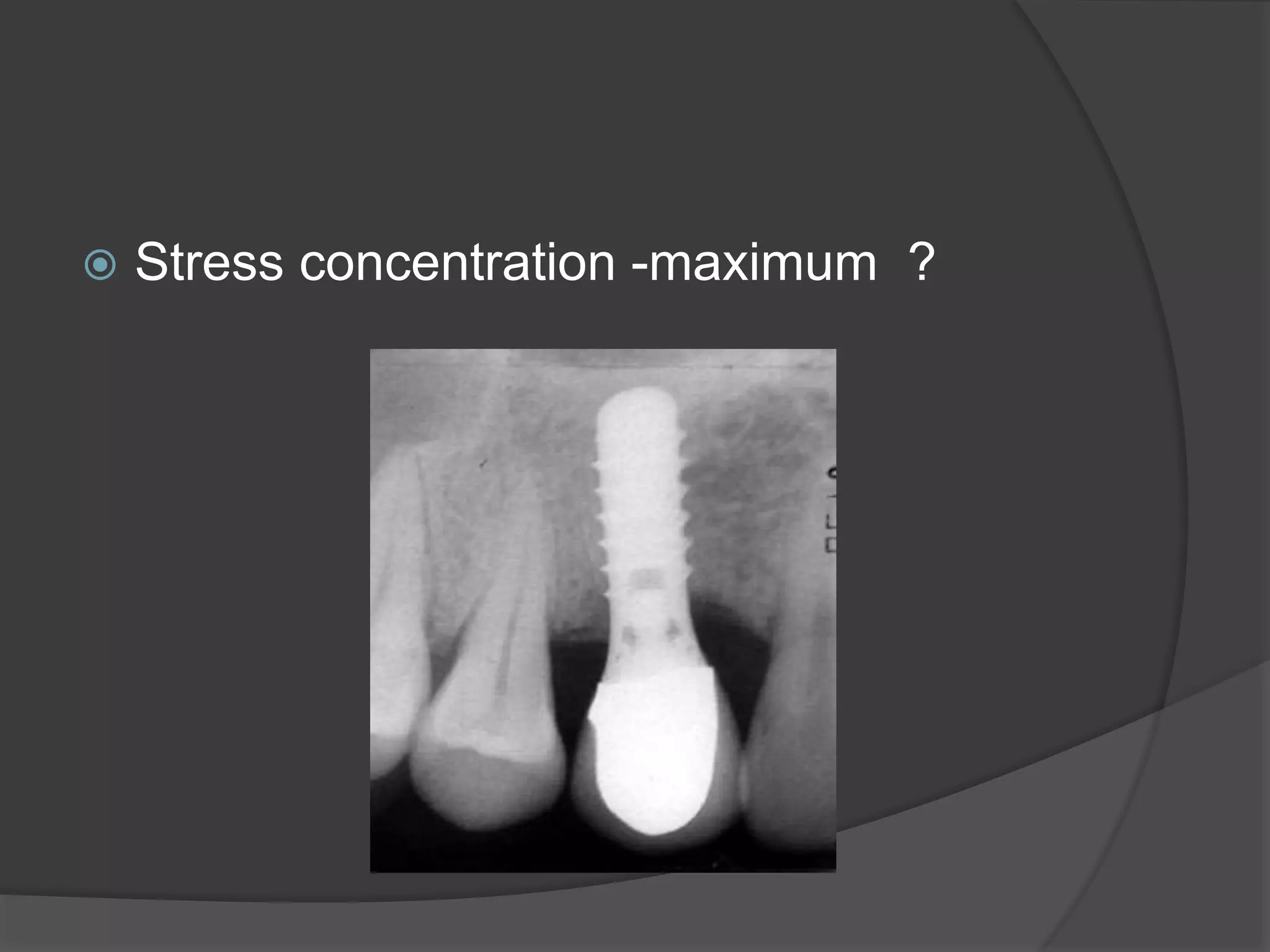
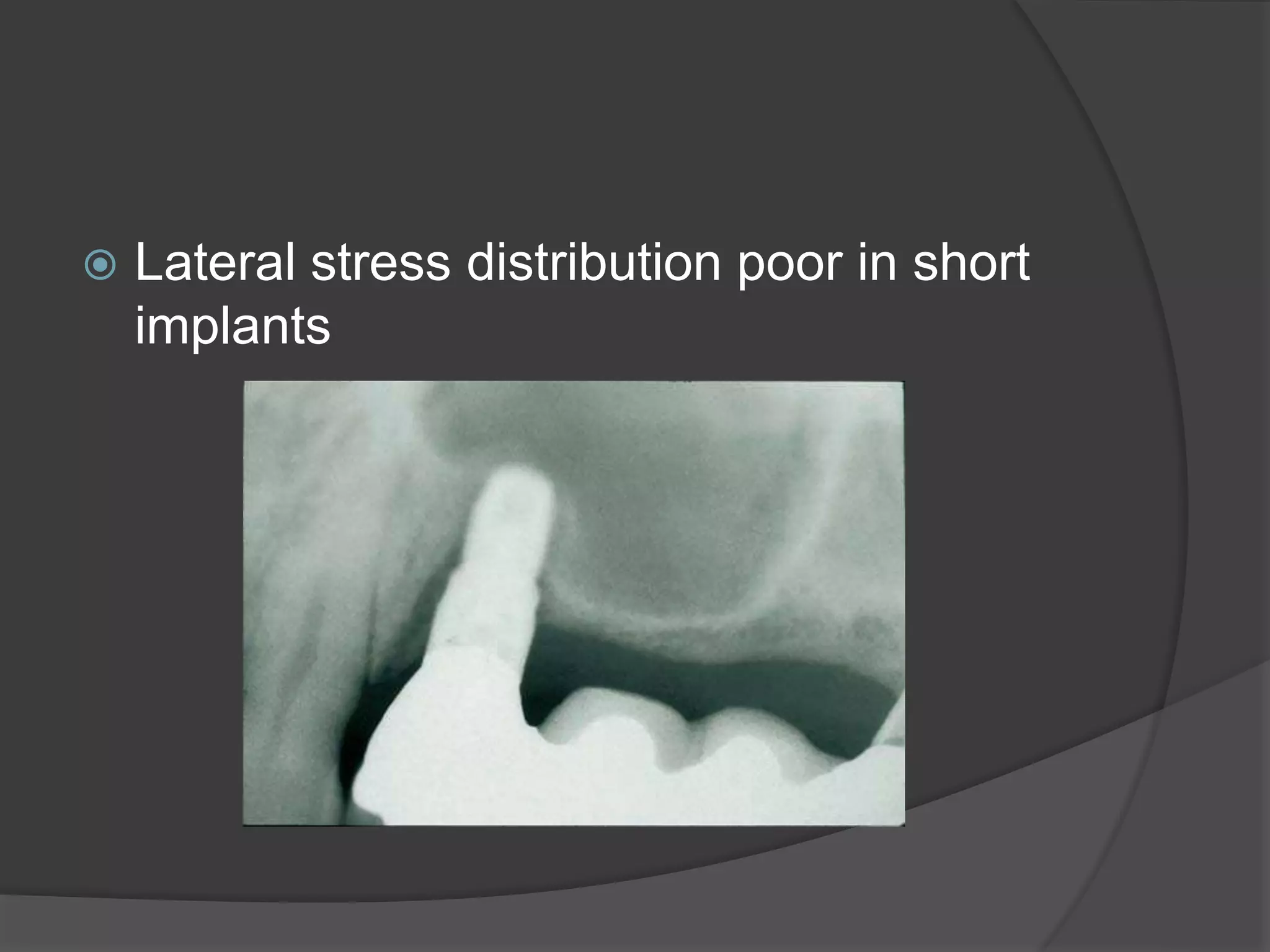
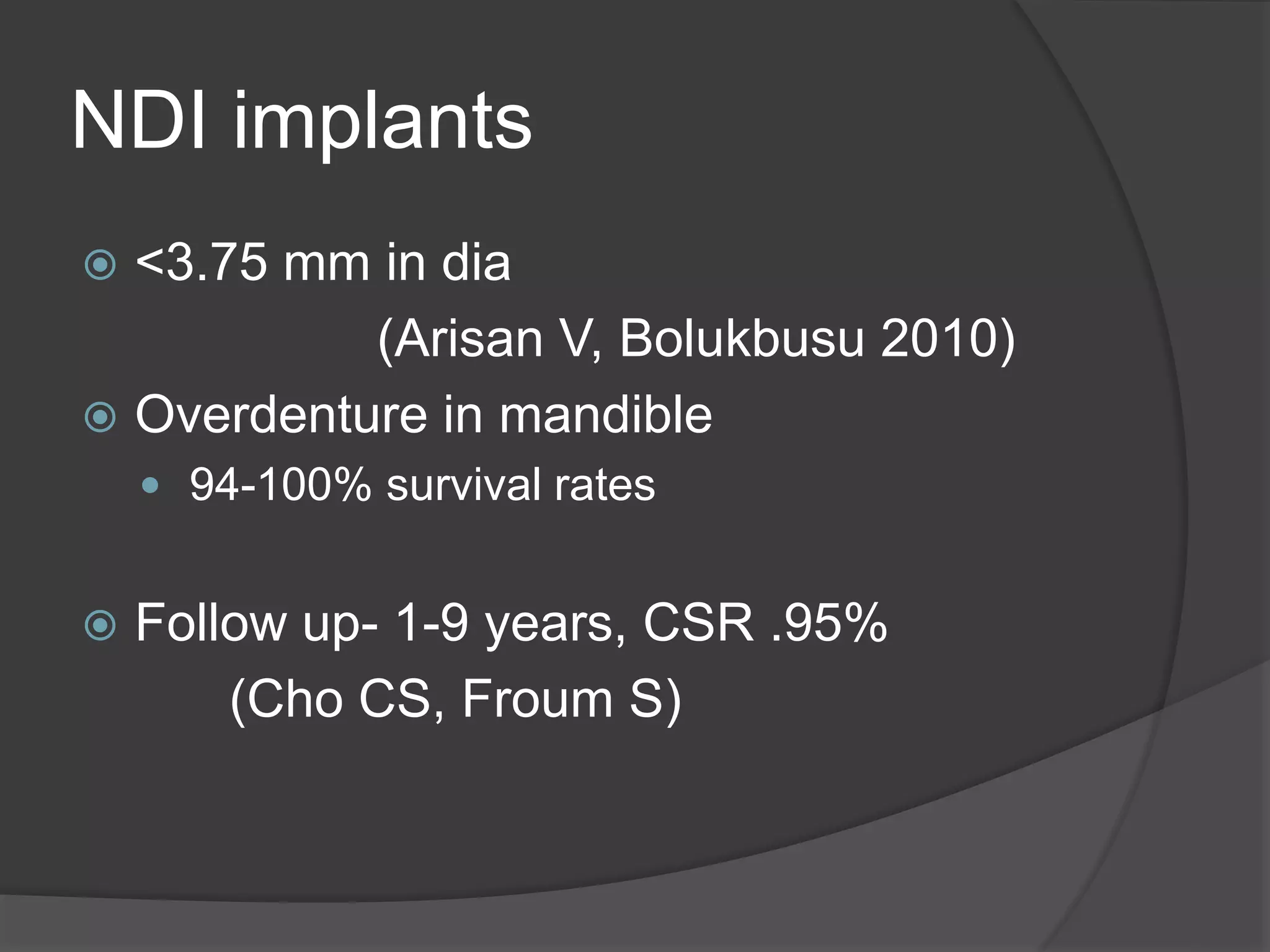
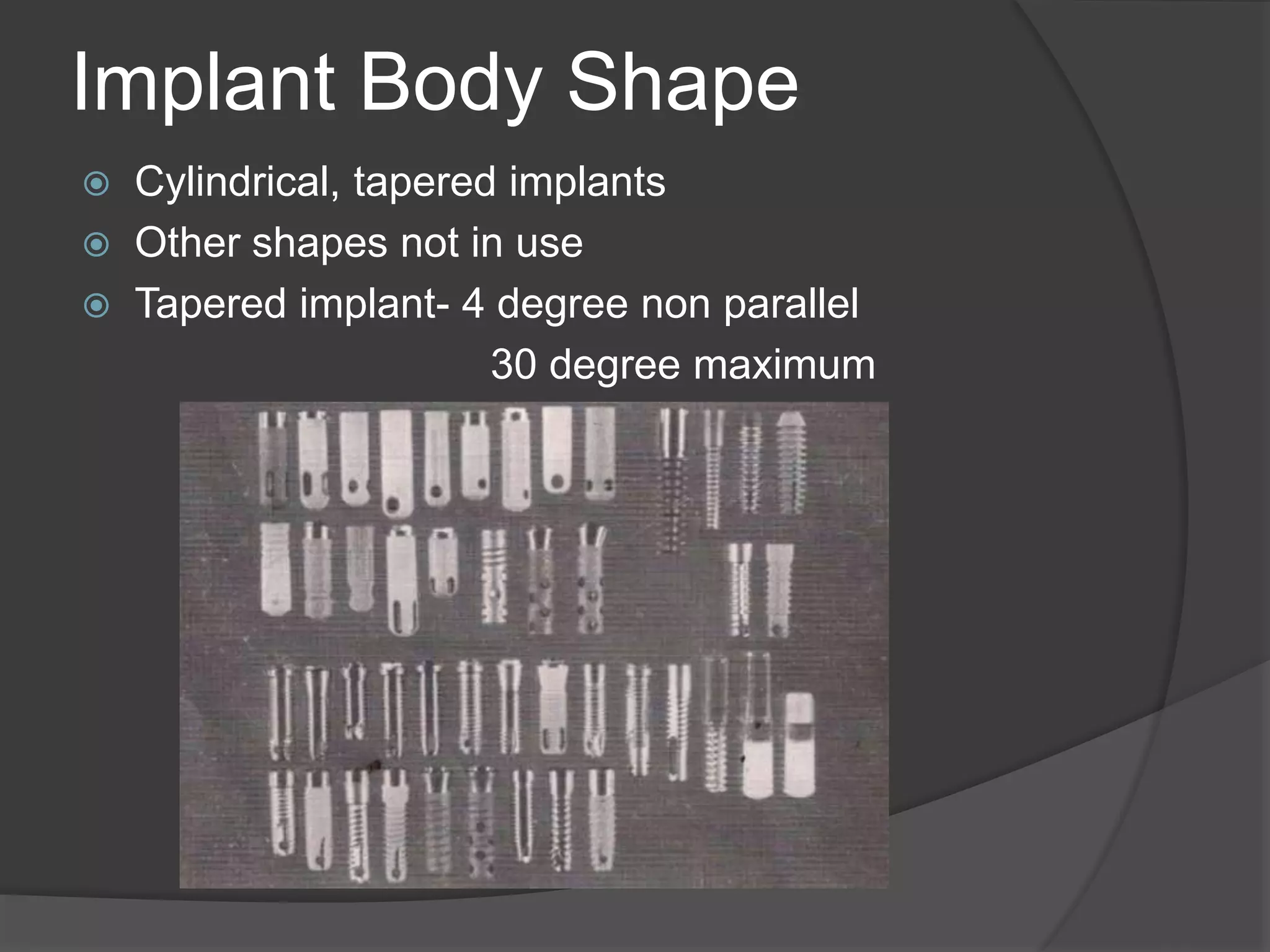
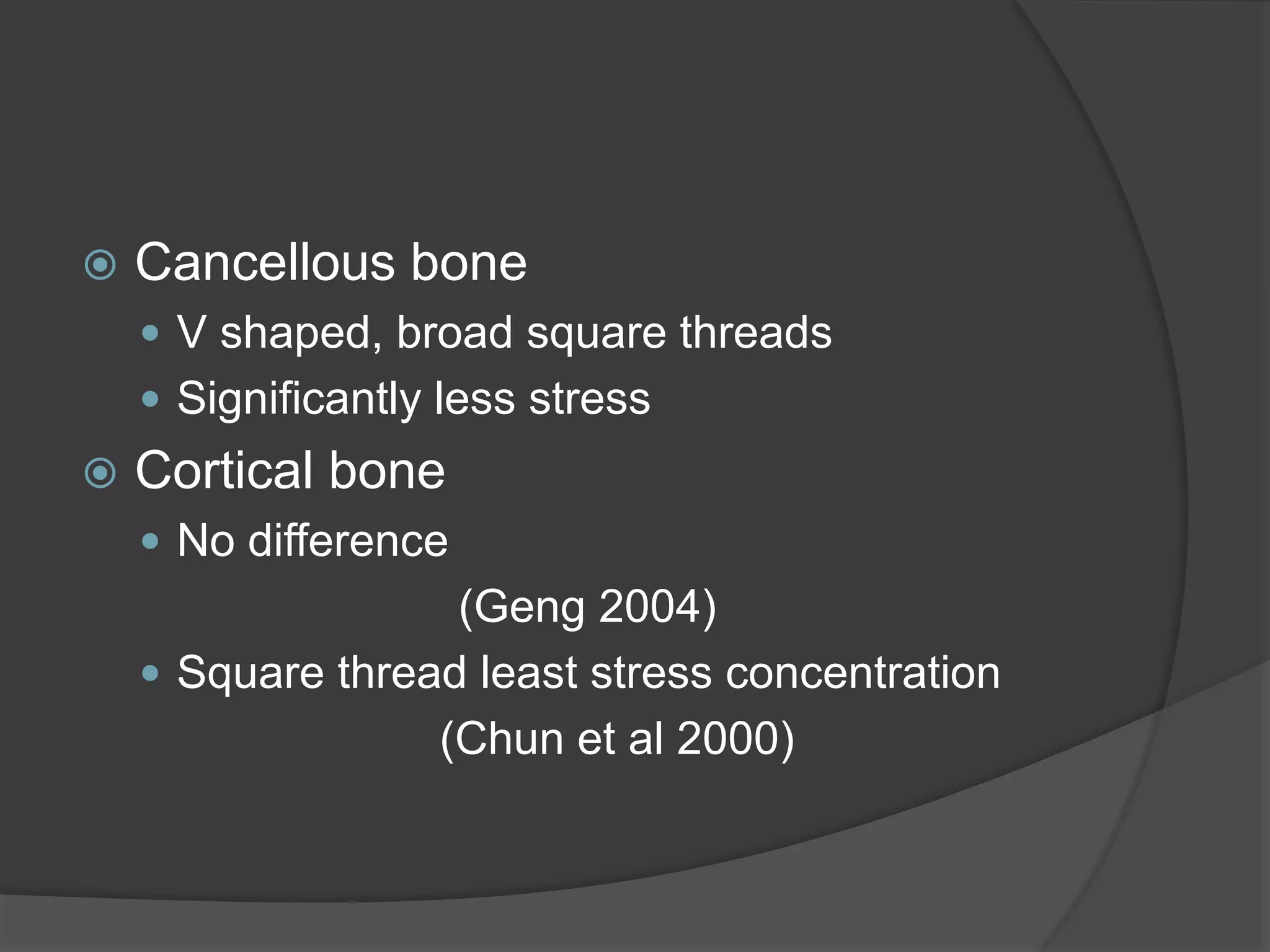
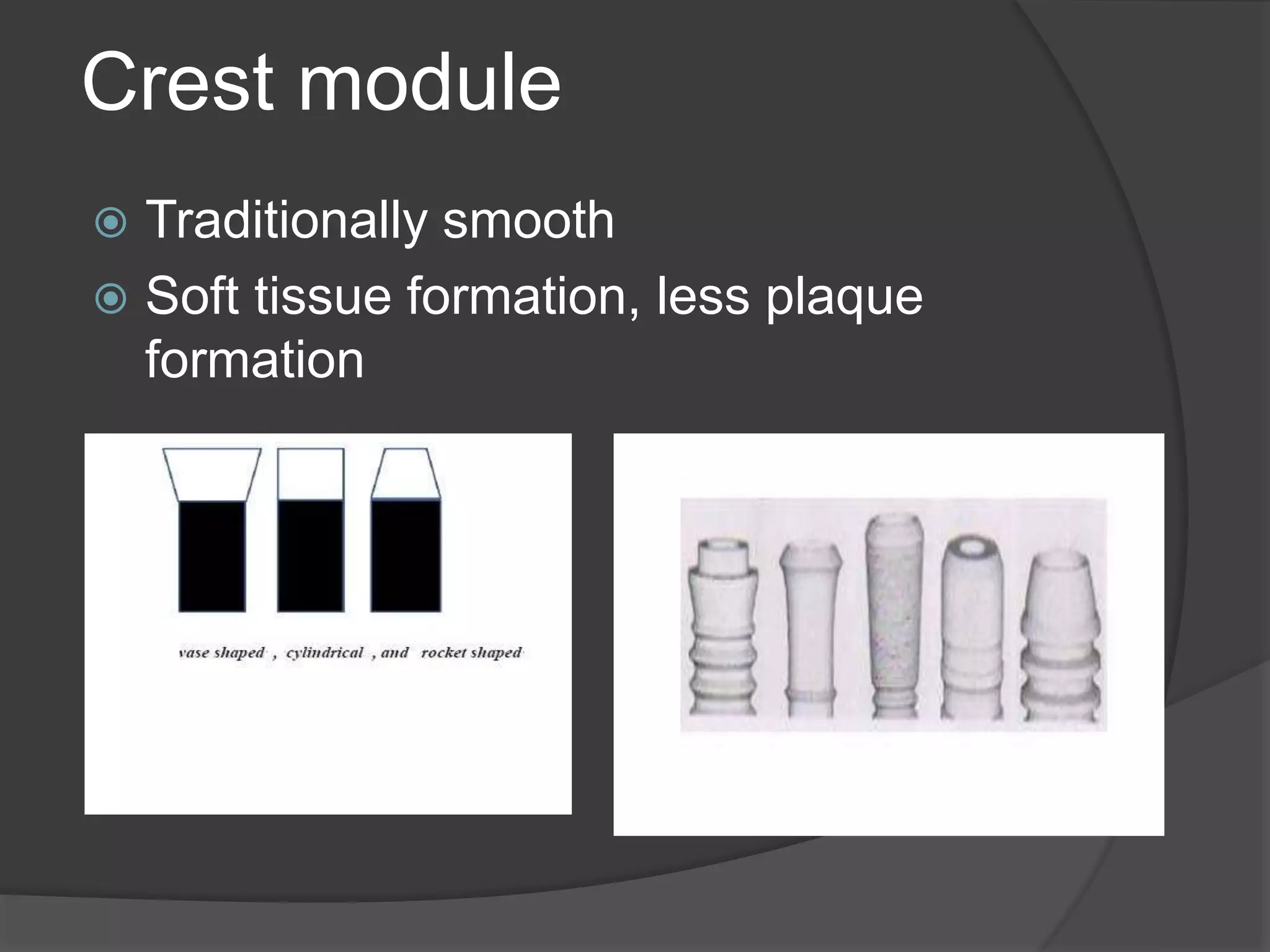
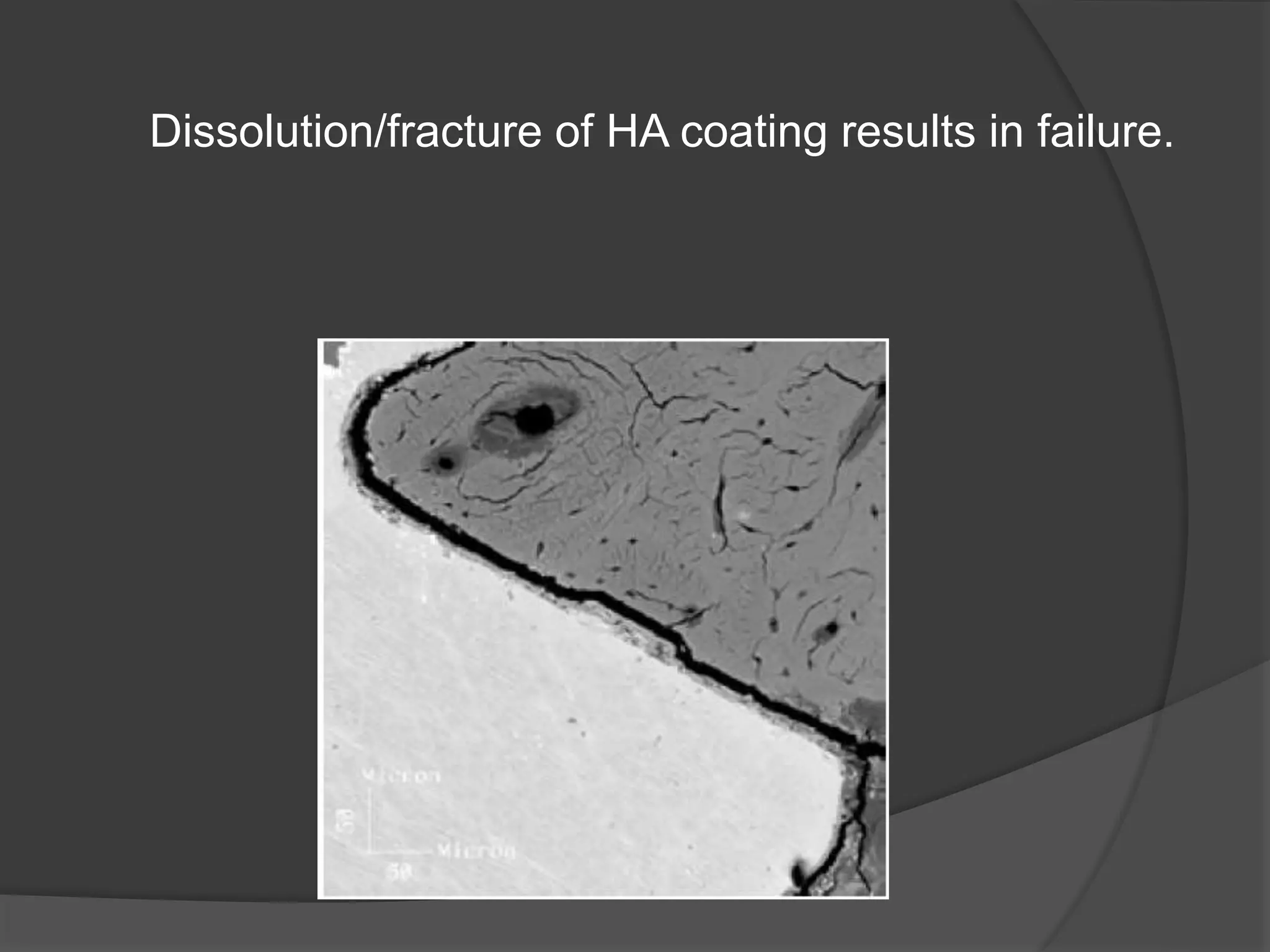
This document discusses factors that influence osseointegration and primary stability of dental implants, including implant design characteristics, surgical technique, and loading protocols. Specifically, it covers the processes of osseointegration and how forces on implants can either promote or inhibit bone remodeling. Key implant design considerations like length, diameter, threads, coatings and surface topography are analyzed in terms of their effects on stress distribution and bone-implant contact. The importance of primary stability and factors influencing it like bone quality and surgical skill are also addressed. Loading protocols ranging from immediate to conventional loading are compared.