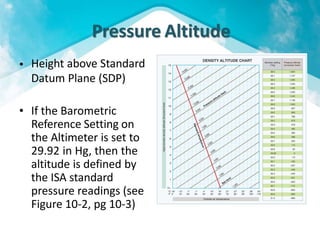
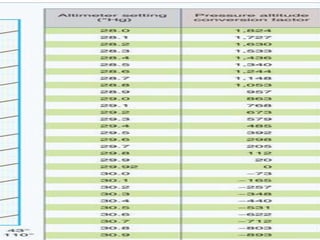
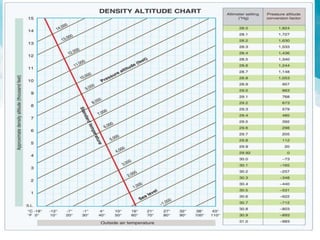
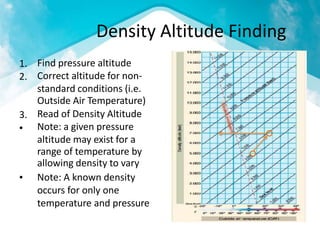
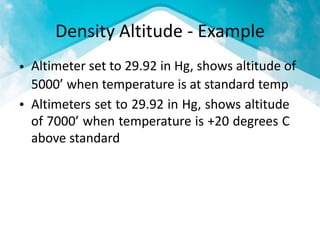
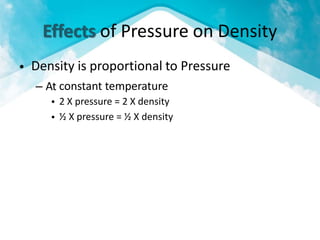
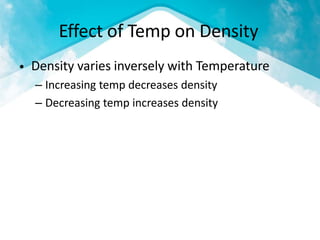
This document discusses atmospheric pressure and its effects on aircraft performance. It describes how atmospheric pressure decreases with altitude due to less air mass above. Pressure and temperature determine air density, with higher density providing better aircraft performance. Density altitude accounts for variations in air temperature, with higher density altitude associated with worse performance. The ideal gas law relates these factors, showing that density decreases with increasing temperature or decreasing pressure. Humidity also reduces air density.