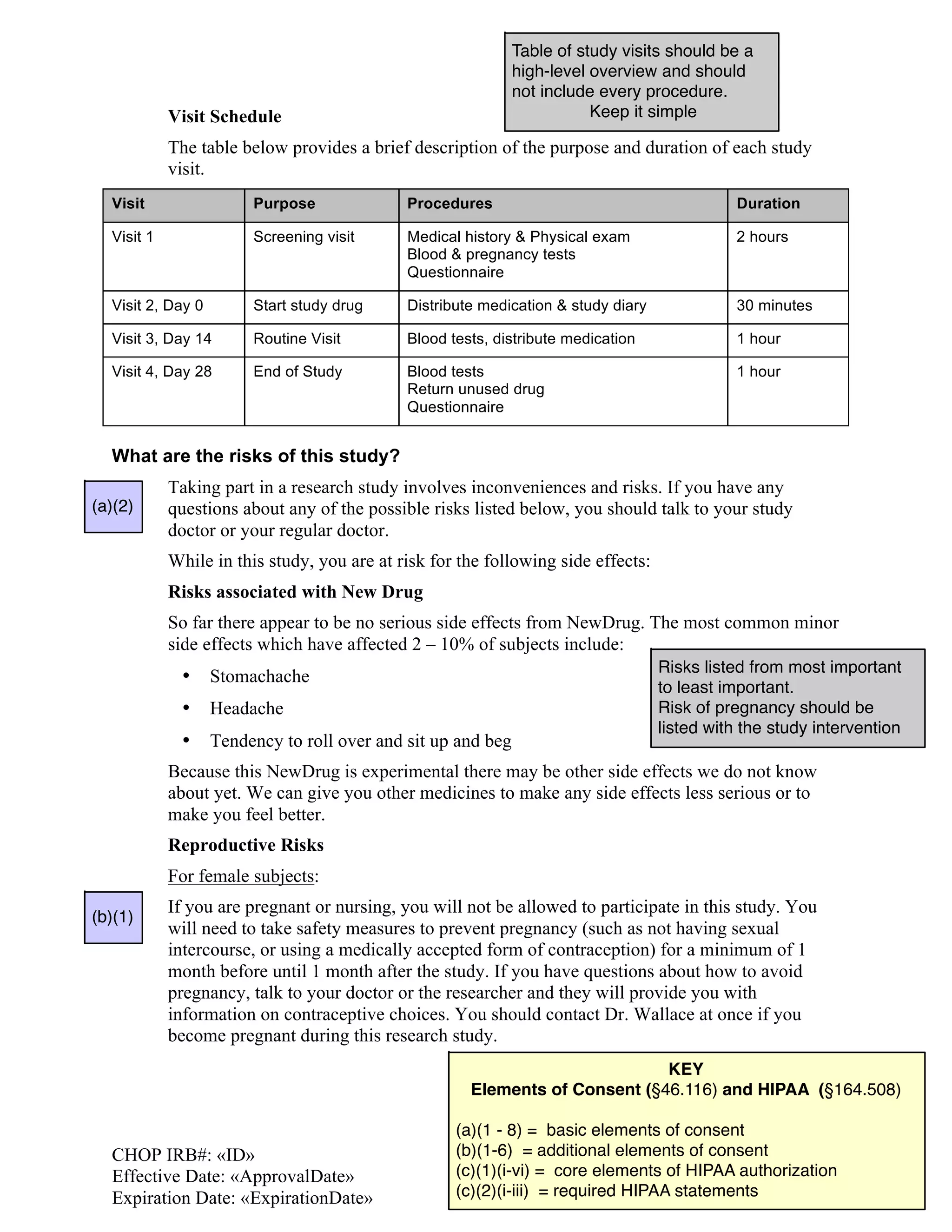
This document is an informed consent form for a clinical trial studying a new drug called NewCure for the treatment of a rare disease in children. The study involves 100 children across 10 medical centers who will receive either NewCure or a placebo over 3 months with 5 clinic visits. Study procedures include medical history interviews, physical exams, blood tests, taking the study drug twice daily for 4 weeks, and completing questionnaires about symptoms before and after treatment. The purpose is to determine if NewCure is safe and effective for treating the rare disease.