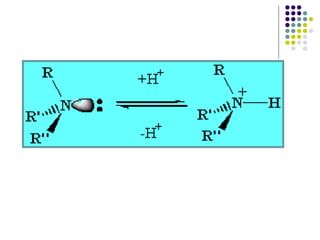
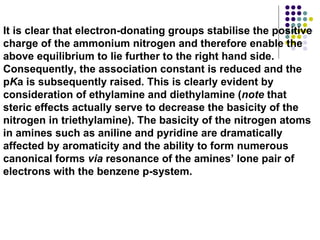
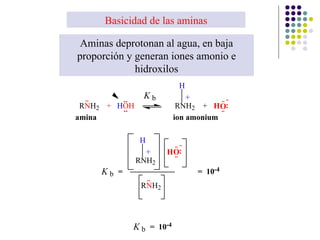
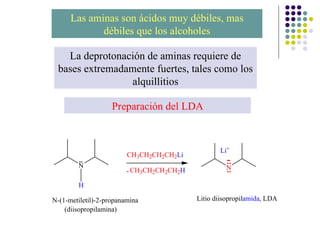
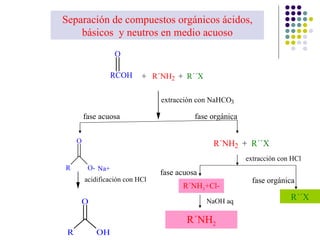
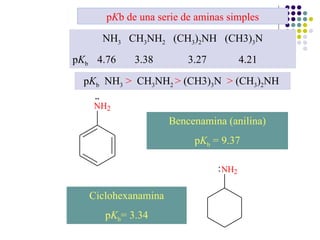
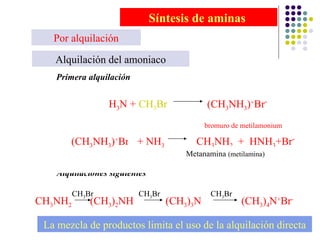
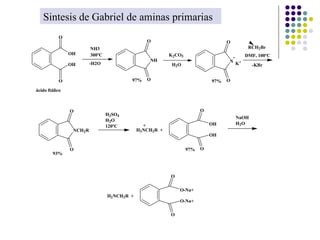
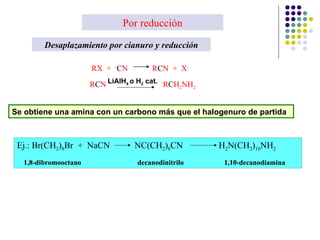
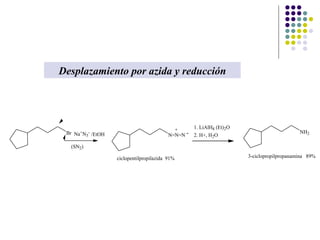
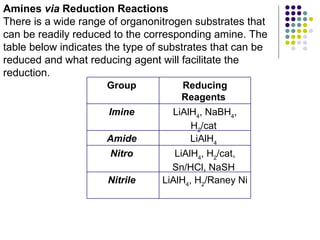
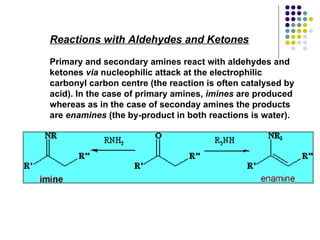
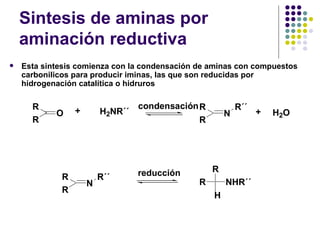
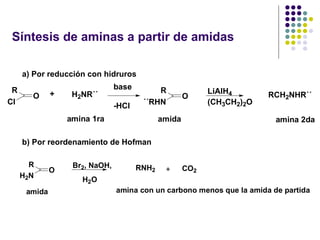
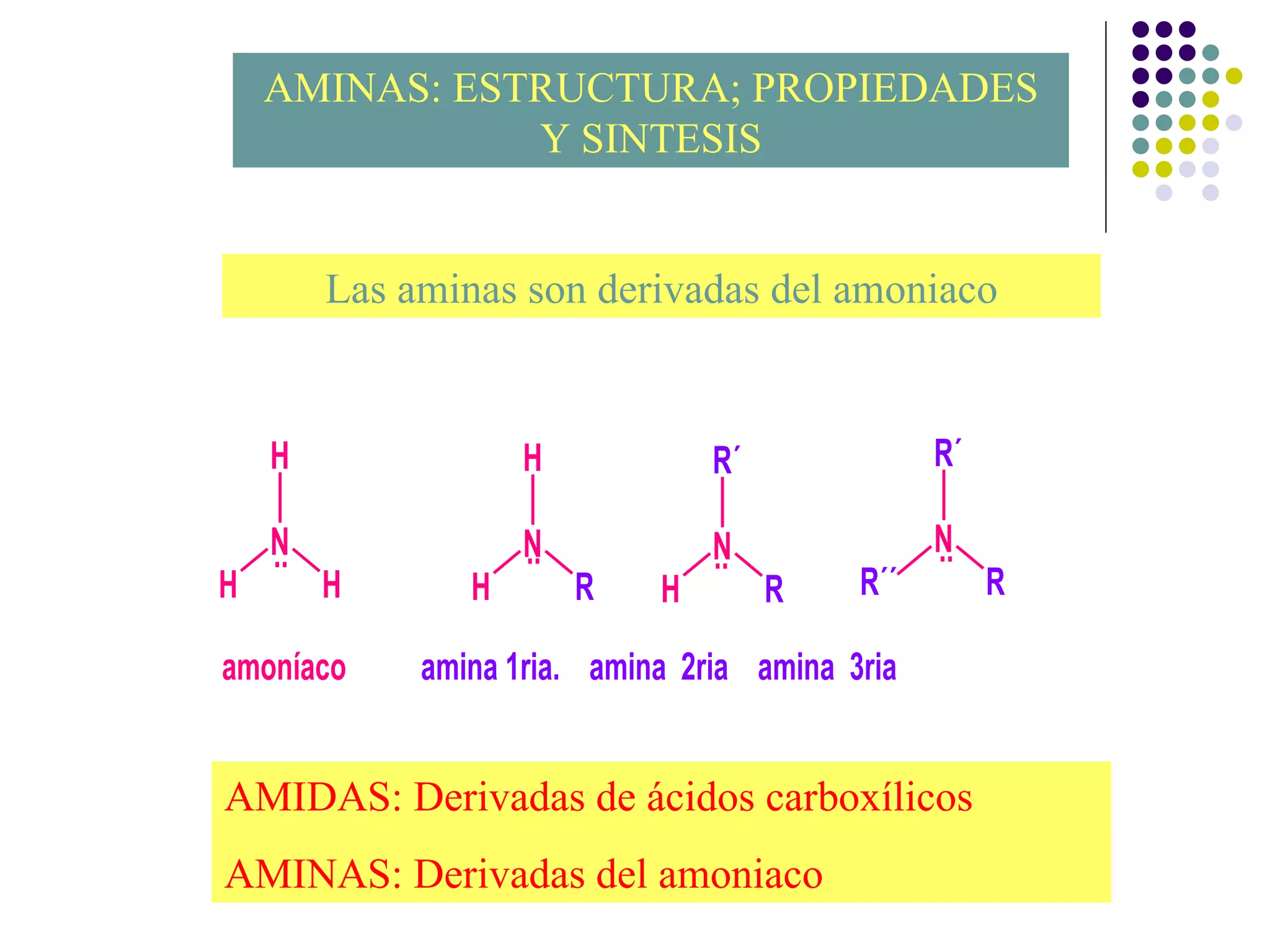
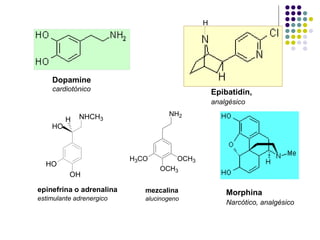
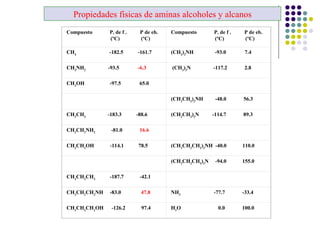
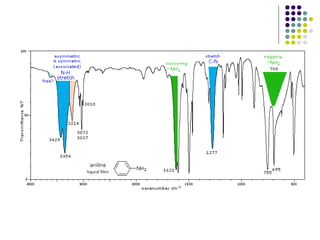
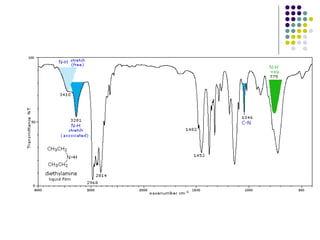
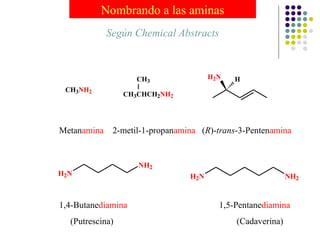
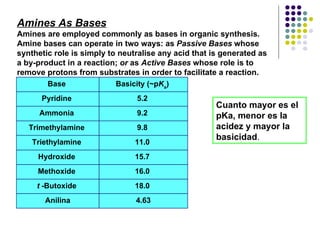
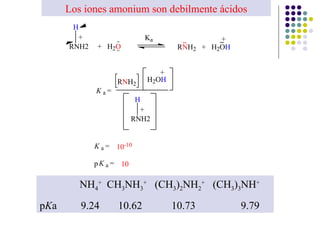
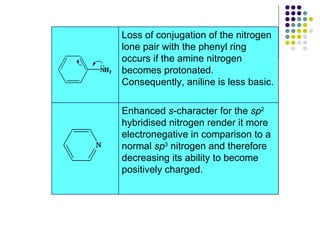
The document discusses the structure, properties, and synthesis of amines. It provides details on the physical properties of amines, alcohols, and alkanes. It also describes the spectroscopic characteristics of the amine group from IR and NMR spectroscopy. The document discusses the pyramidal structure of amines and why they are not chiral or optically active due to rapid nitrogen inversion. It covers acid-base properties of amines and how they can act as both acids and bases. Finally, it outlines several methods for synthesizing amines, including alkylation, Gabriel synthesis, cyanide displacement/reduction, azide displacement/reduction, and reductive amination.


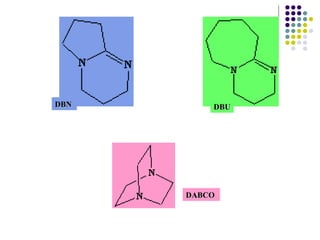
![The passive bases (in general the weaker bases) are employed in reactions that require a base in order to attain completion. An example of this is the acylation of primary or secondary amines. In these reactions, bulky, non–nucleophilic bases such as Hünig’s base (diisopropylethylamine) are frequently used. More powerful amine bases are required when the reaction involves removal of a proton from a substrate in order to generate an anionic intermediate. The most commonly employed strong organonitrogen base is lithium diisopropylamide (LDA) (a metal amide) – this is produced by treating diisopropylamine with butyl lithium. Reactions using LDA are typically carried out at –78 ºC in order to avoid detrimental side reactions. In the case of substrates where two acidic protons can be abstracted by base, treatment with LDA leads to the kinetic (i.e. sterically least hindered ) product. LDA is not as strong a base as BuLi, however, it is too hindered to act as a nucleophile. In the uncommon case where it does participate as a nucleophile, LDA can be replaced by the even bulkier base, lithium hexamethyldisilazide. Strong bases such as LDA are not always required to abstract protons from substrates. In the case of elimination reactions, milder organonitrogen bases such as 1,5-diazabicyclo[4.3.0]non-5-ene (DBN), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 1,4- diazabicyclo[2.2.2]octane (DABCO) or even pyridine are employed.](https://image.slidesharecdn.com/aminas-2207/85/Aminas-20-320.jpg)