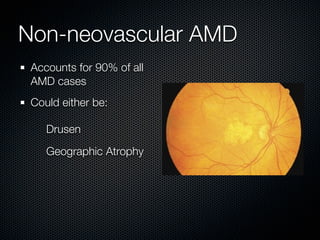
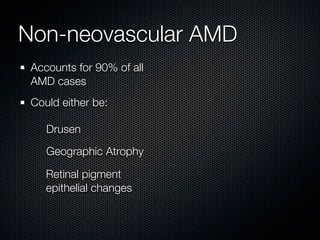
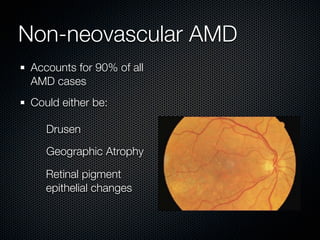
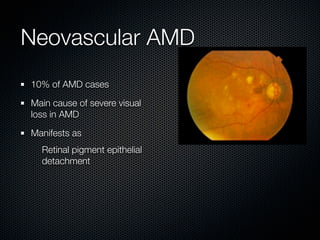
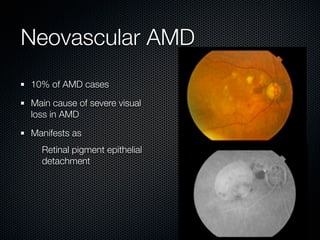
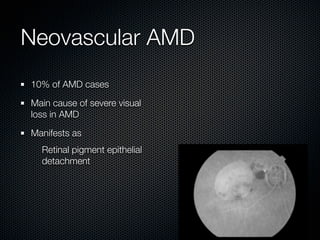
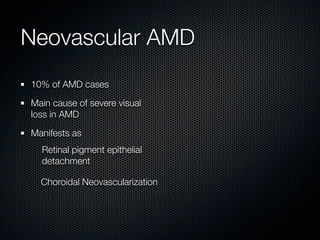
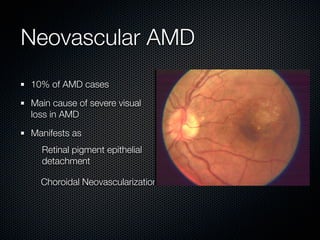
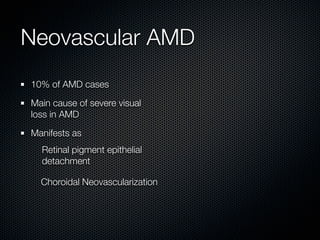
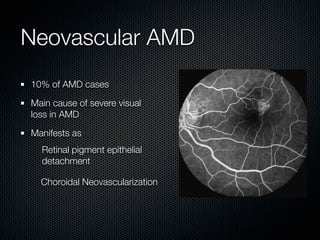
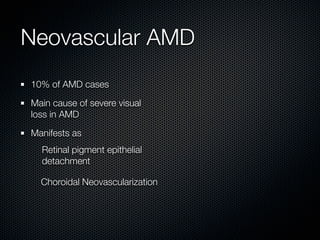
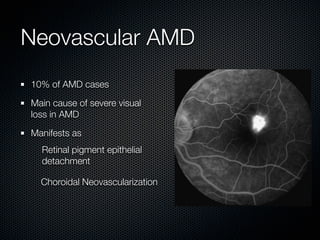
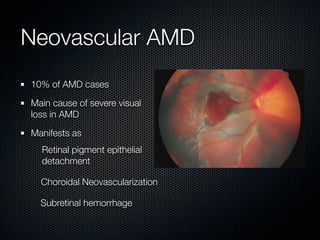
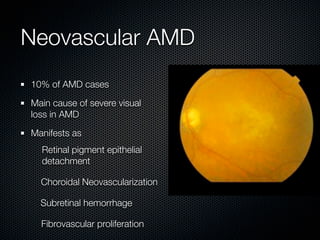
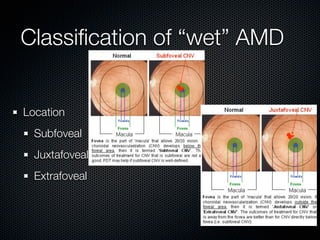
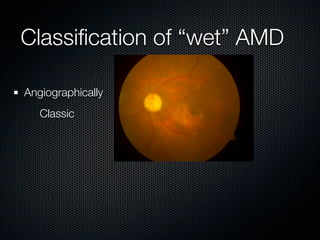
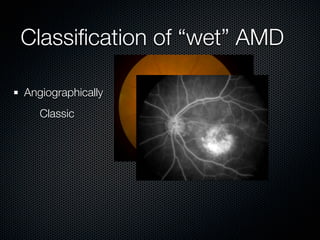
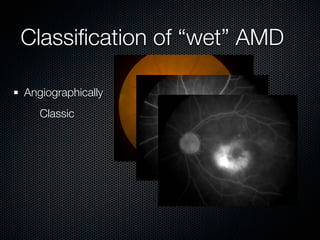
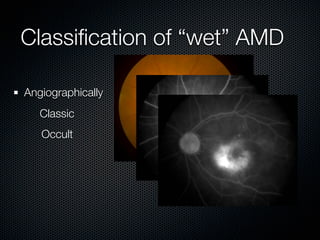
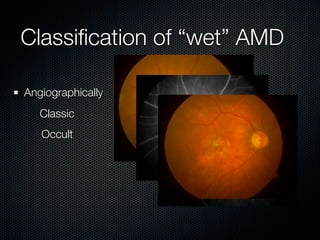
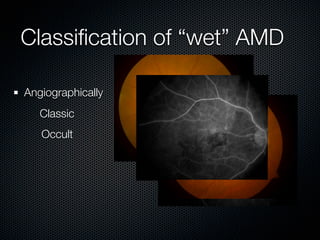
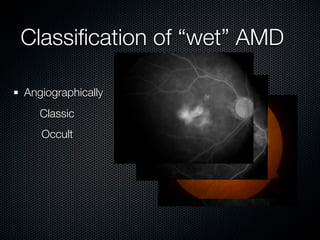
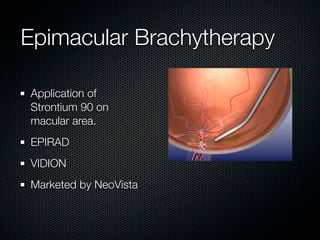
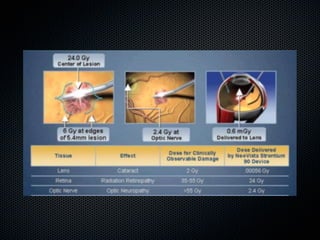
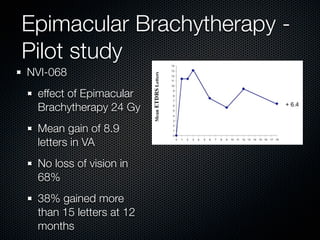
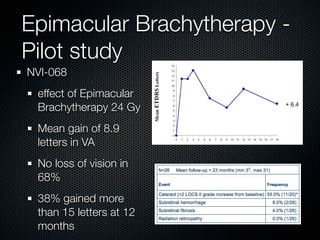
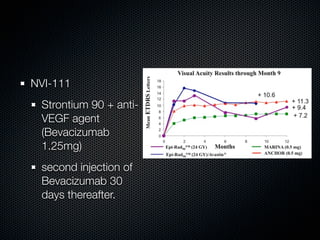
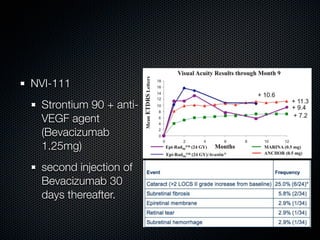
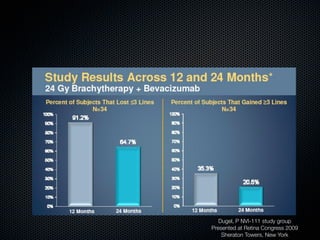
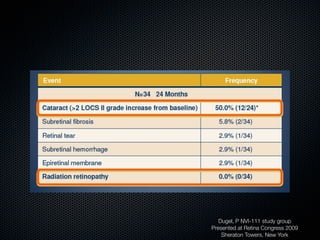
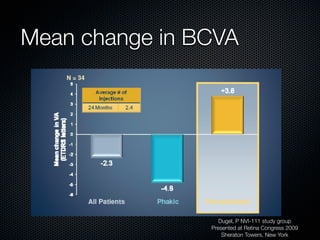
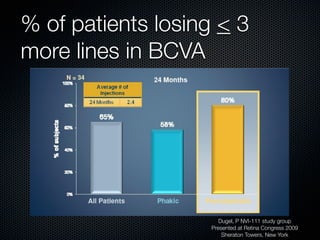
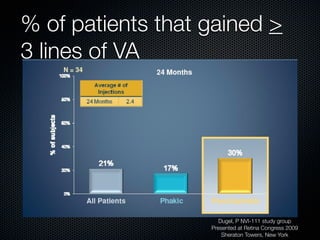
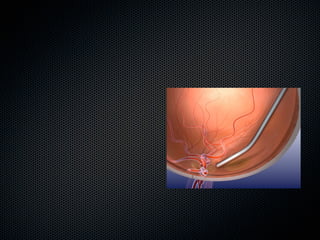
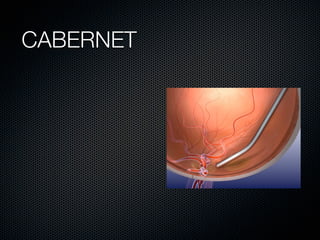
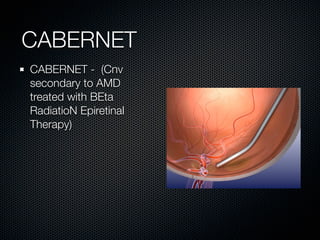
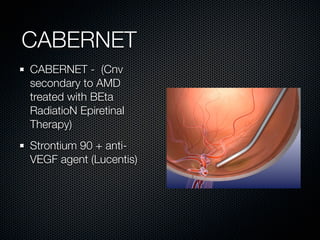
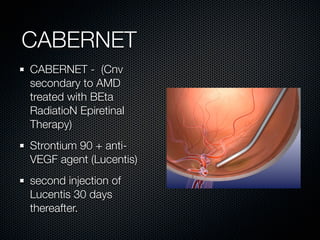
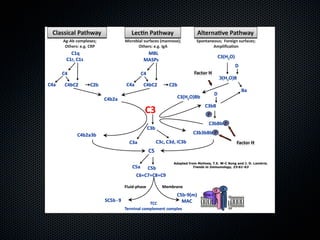
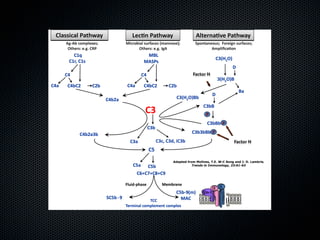
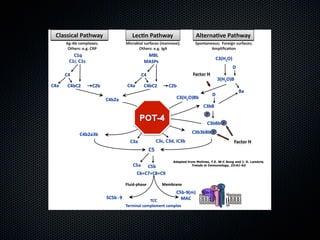
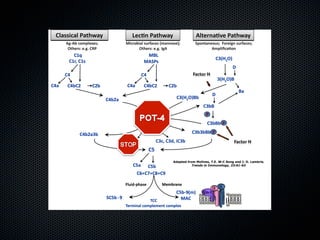
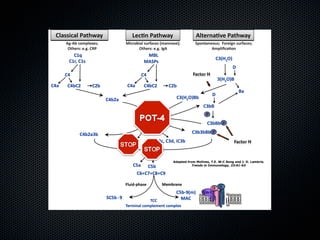
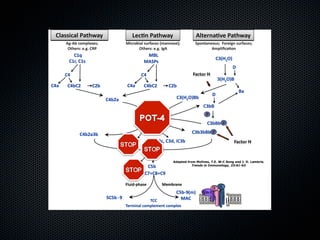
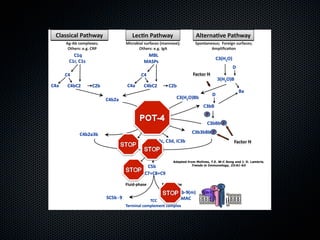
This document summarizes emerging treatments for neovascular age-related macular degeneration (AMD). It discusses that AMD is a progressive eye disease where photoreceptors are lost. There are two main types: neovascular (wet) AMD and non-neovascular (dry) AMD. For wet AMD, treatments discussed include ranibizumab, photodynamic therapy, and combination therapies using anti-VEGF drugs with PDT and steroids. Ongoing clinical trials are exploring combination therapies. The document reviews past and current research on treatments seeking to prevent vision loss from wet AMD.