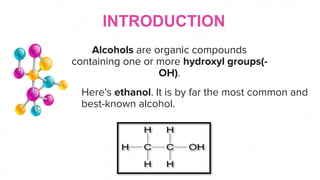
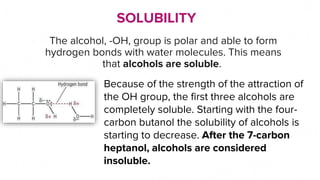
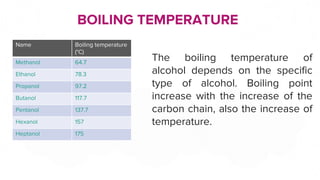
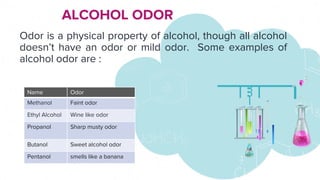
This document discusses the physical properties of alcohols including their solubility, boiling points, melting points, viscosity, odor, and flammability. It explains that alcohols are soluble due to their polar hydroxyl groups which allow hydrogen bonding with water molecules. Solubility decreases with longer carbon chains over 7 carbons. Boiling and melting points increase as the carbon chain lengthens. Viscosity increases with longer chains and decreases with higher temperatures due to weaker hydrogen bonding. Odor varies between alcohols from faint to wine-like to sharp or sweet. Flammability also depends on molecular structure but generally alcohols are flammable due to their carbon and hydrogen content.