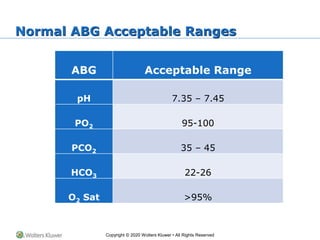
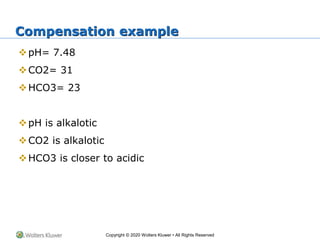
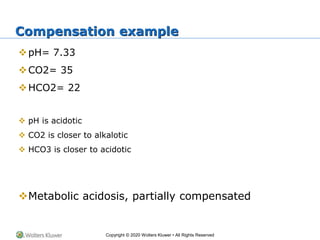
The document outlines the fundamentals of acid-base balance in the body, emphasizing the roles of major organs such as the lungs and kidneys in maintaining normal pH levels (7.35-7.45). It details the types of acidosis and alkalosis, the parameters measured in arterial blood gas (ABG) analysis, and how to interpret these results to identify single and double disorders. Additionally, it discusses compensation mechanisms that the body employs to correct pH imbalances.