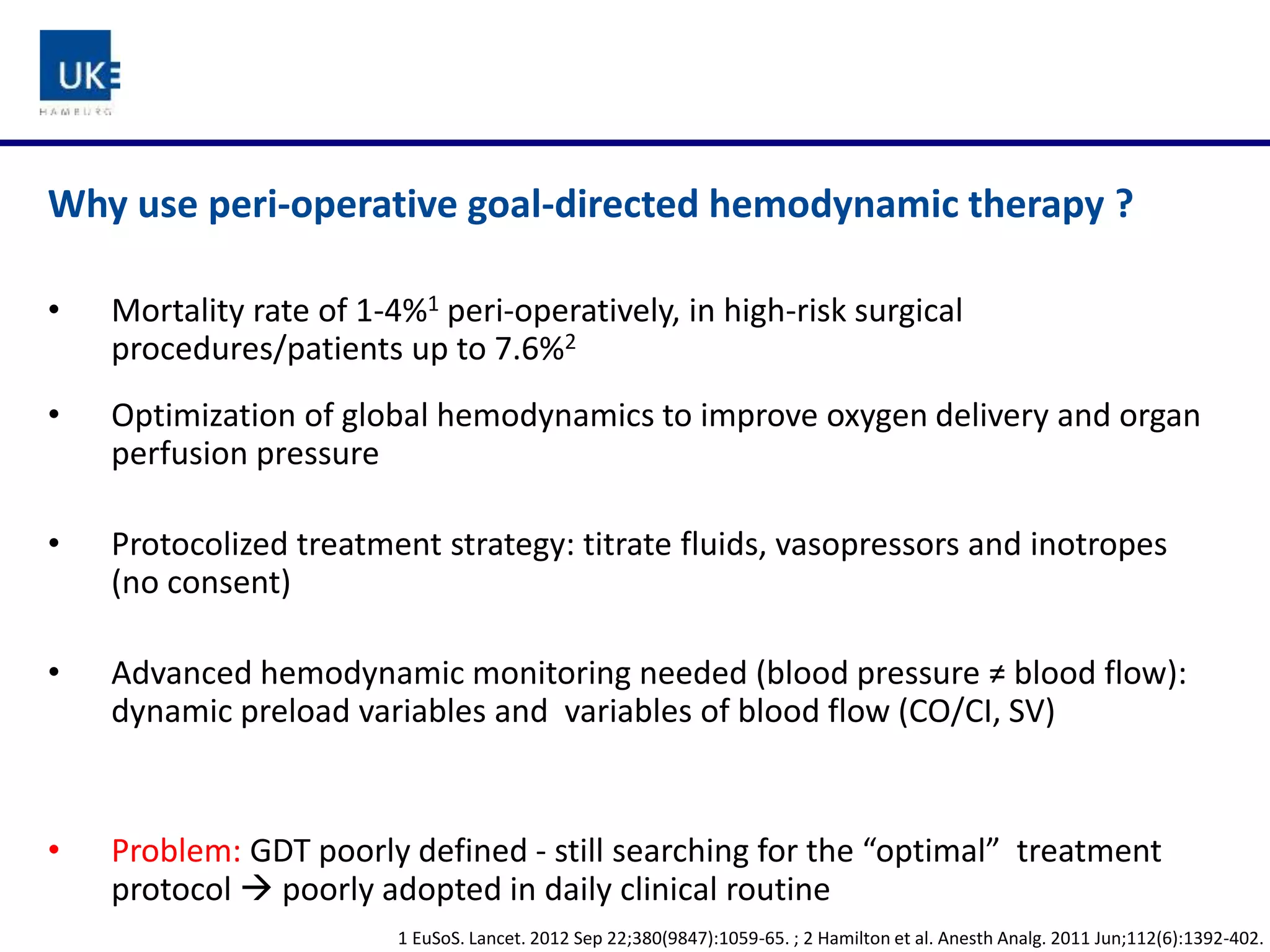
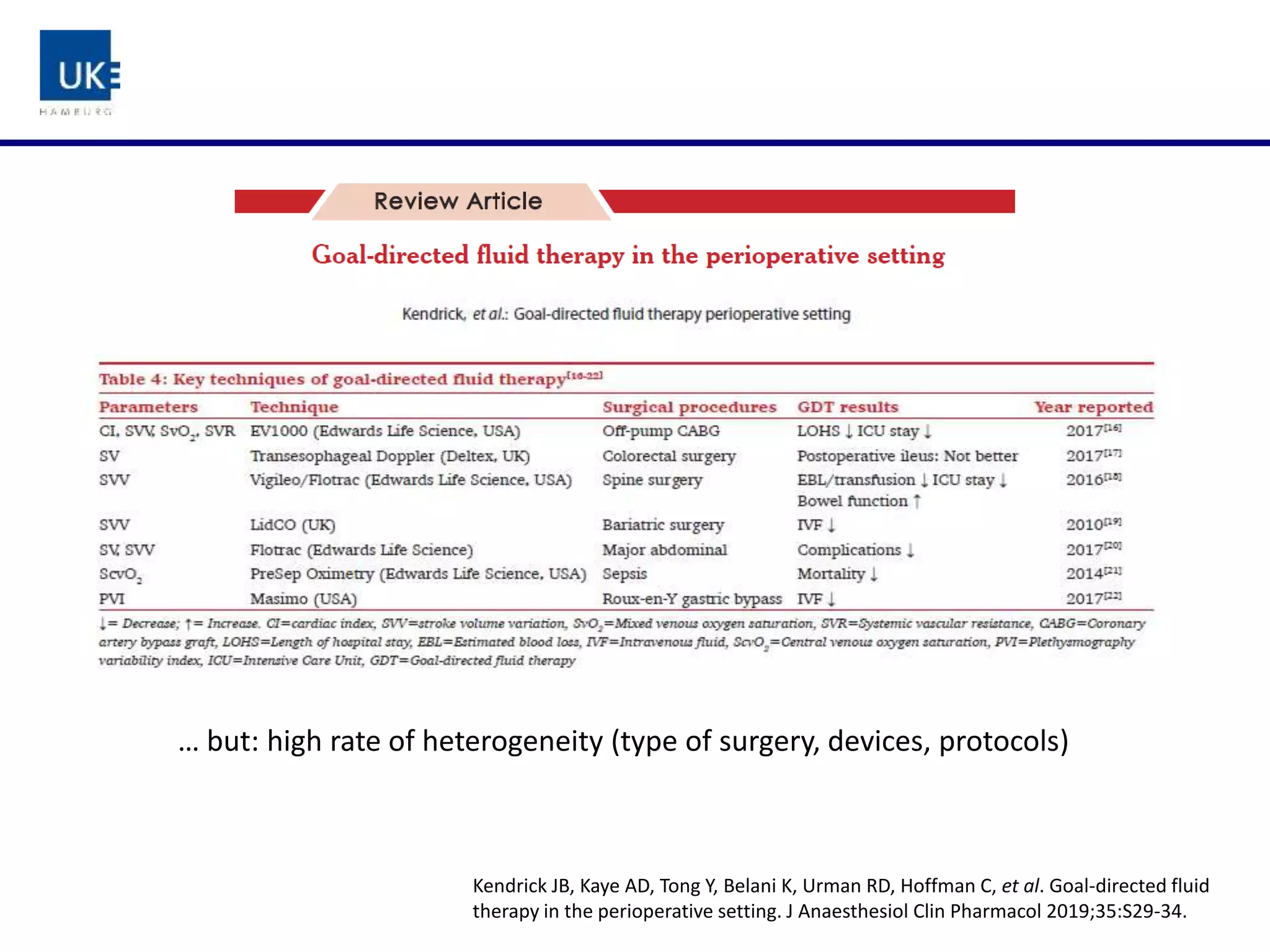
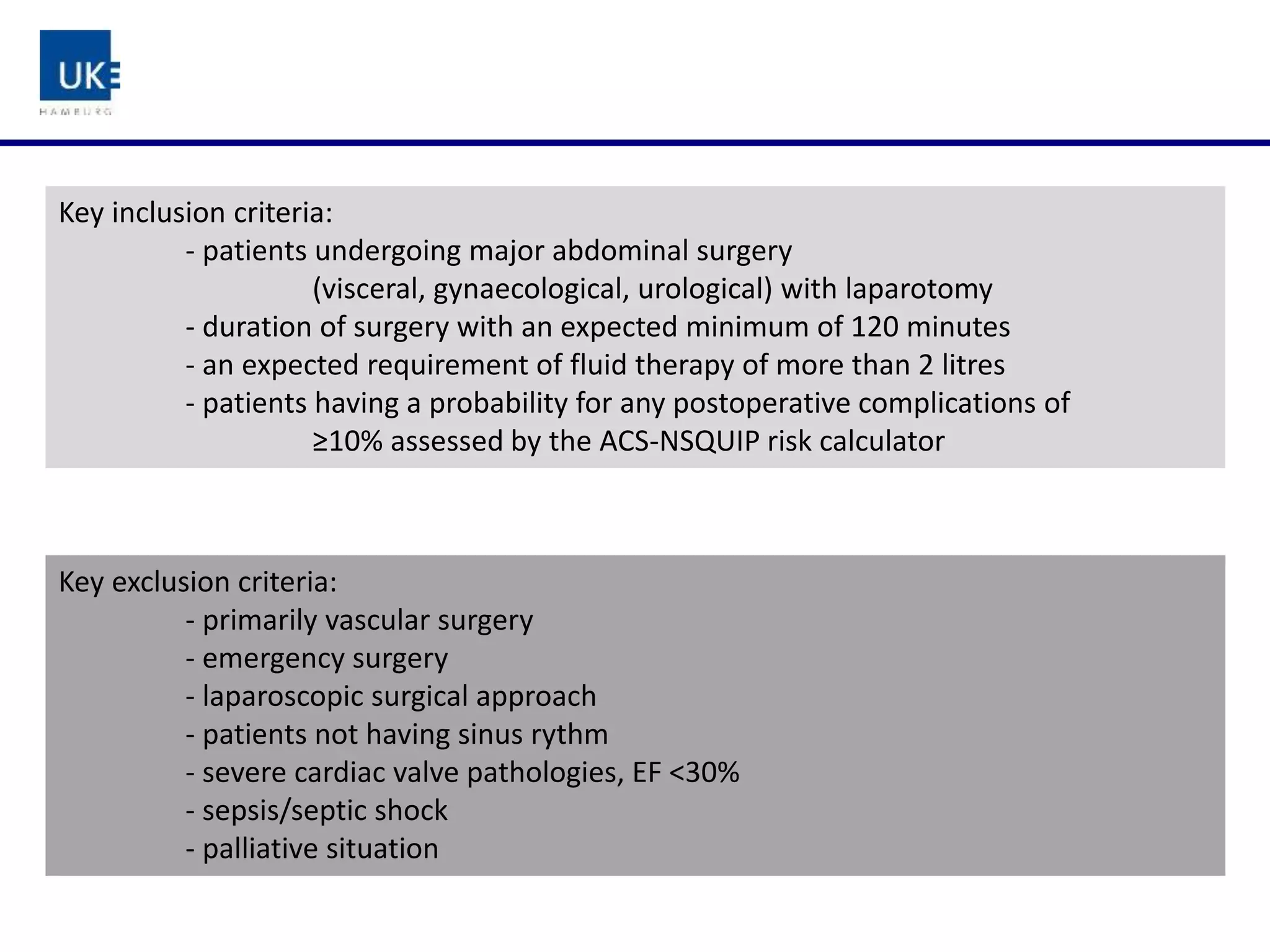
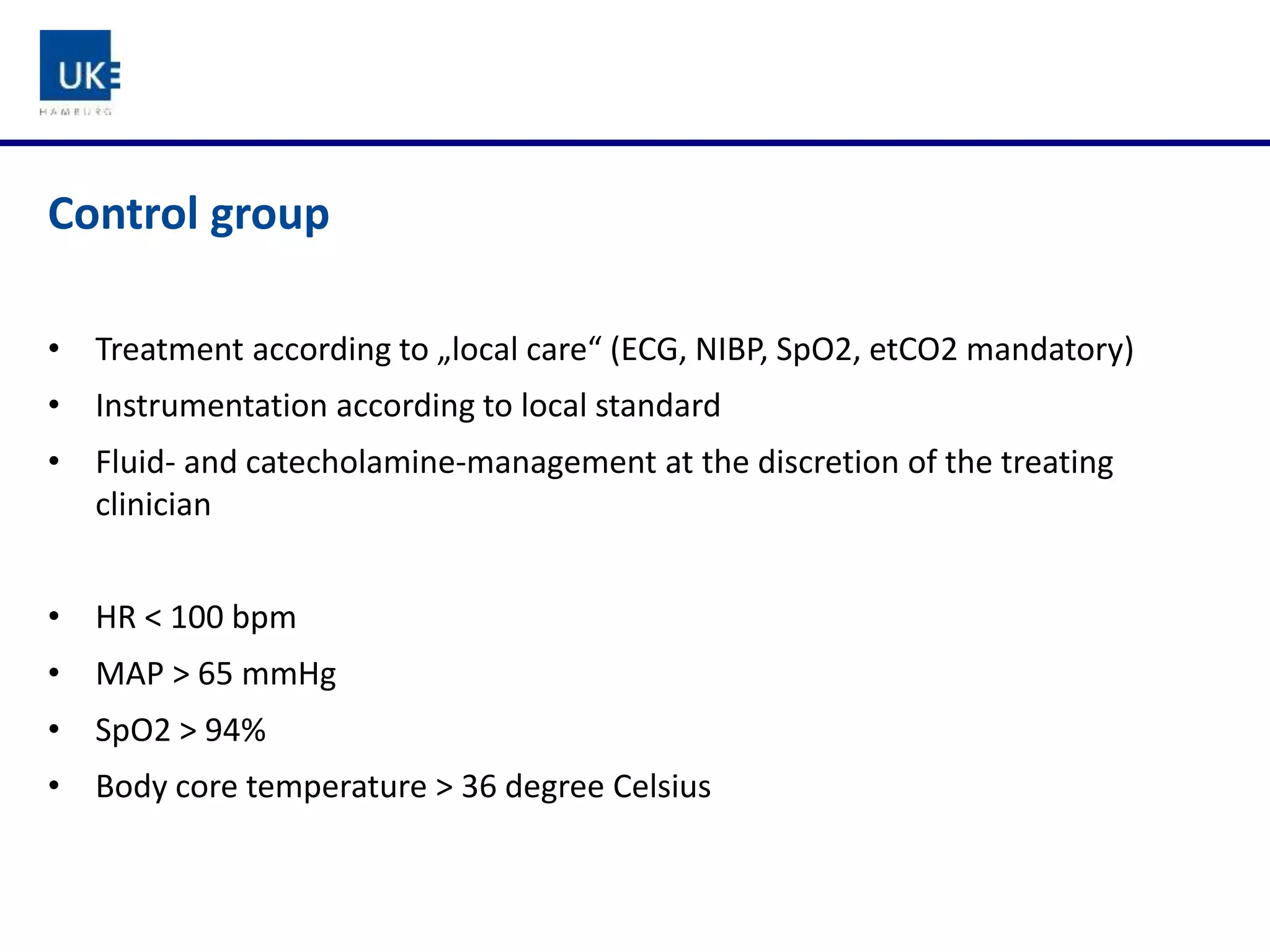
The document discusses a study on goal-directed hemodynamic therapy (GDT) aimed at improving outcomes for high-risk surgical patients. It highlights the need for advanced monitoring and the variability in GDT protocols, as well as the design and progress of the ongoing trial with interim results indicating a high occurrence of complications. The authors conclude that GDT can be beneficial for peri-operative risk management, necessitating more large-scale, multicenter trials.














![Sample Size Calculation
Incidence of primary endpoint in the intervention group: 33%
Incidence of primary endpoint in the control group : 48%
Maximum
Test significance level, a 0.0500
1 or 2 sided test? 2
Group 1 proportion, p1 0.48
Group 2 proportion, p2 0.33
Odds ratio, y = p2 (1 - p1) / [p1 (1 - p2)] 0.5336
Power ( % ) 80.00
n per group 167
Estimated drop-out rate of 10% and
exclusion of the first 2 patients per centre
(n=6)
n=380 Patienten](https://image.slidesharecdn.com/9-191028173223/75/9-ifad2019-review-of-recent-fluid-trials-funcke-24-2048.jpg)
