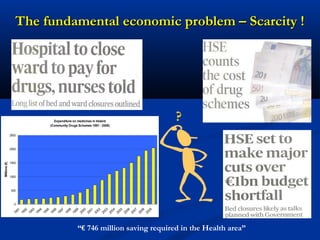
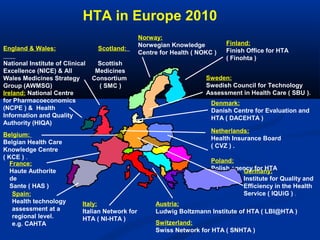
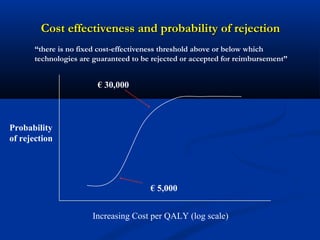
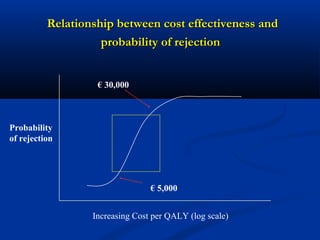
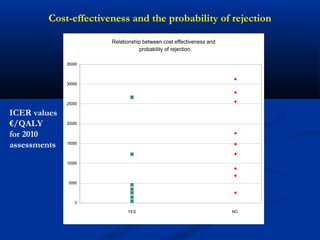
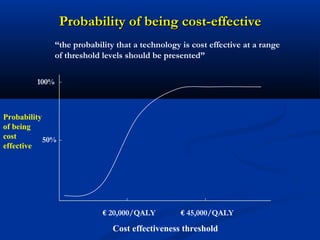
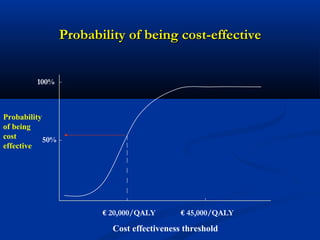
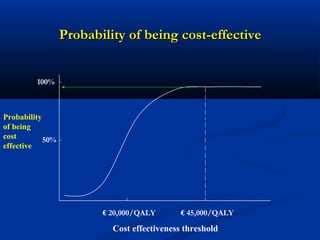
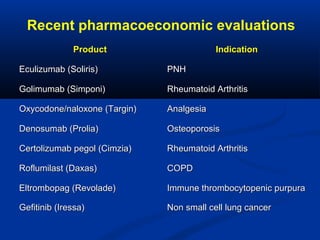
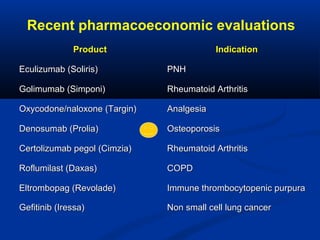
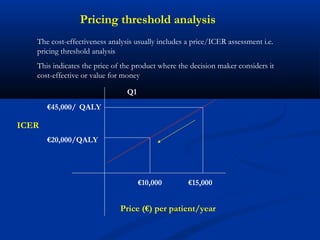
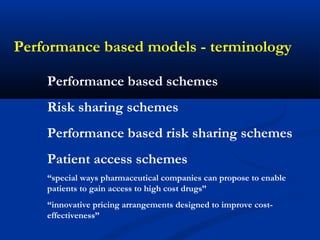
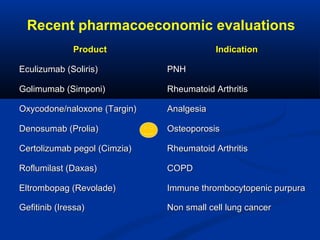
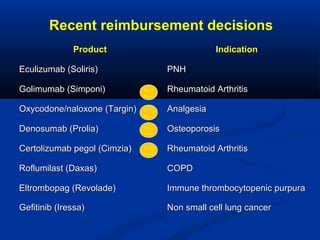
The document discusses the role of health technology assessment (HTA) in Ireland, emphasizing its importance for ensuring optimal health outcomes while managing costs. It highlights recent developments in HTA processes, including cost-effectiveness evaluations for new pharmaceutical products, and the increasing sophistication of assessment methods. Furthermore, it addresses challenges like budget constraints and the need for innovative pricing and risk-sharing agreements for high-cost drugs.