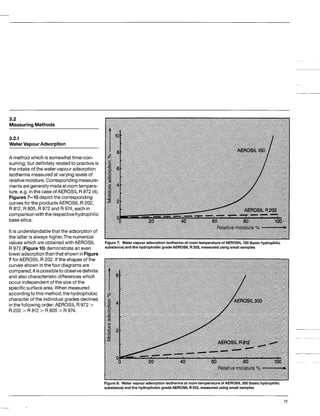
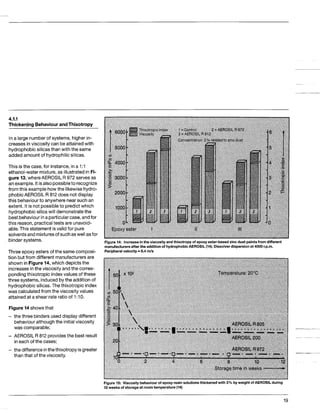
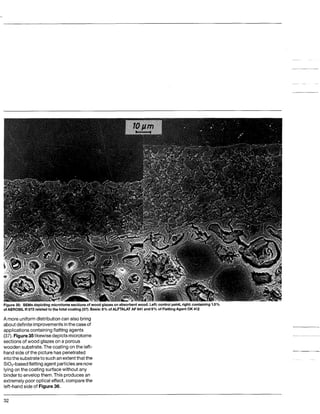
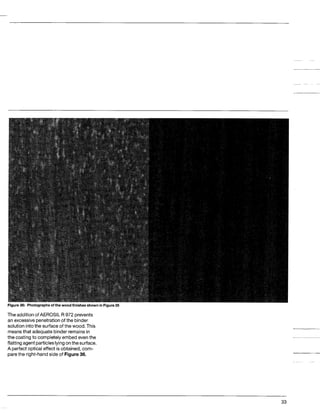
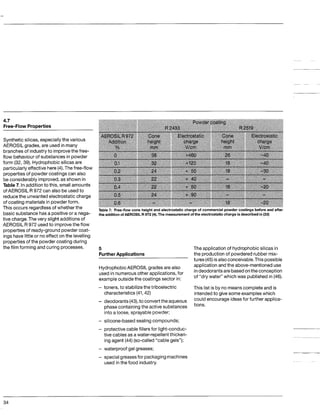
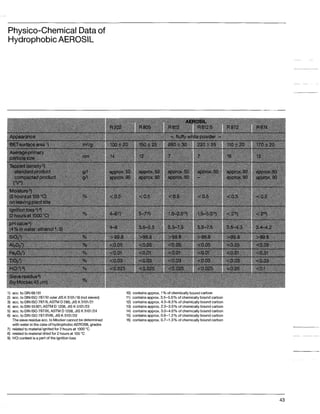
This document discusses methods for measuring the hydrophobicity of various AEROSIL products. It begins with an overview of how AEROSIL R 972 was the first industrially produced hydrophobic silica in 1962. Several new hydrophobic AEROSIL grades have since been developed with different surface treatments and starting materials. The document then examines various techniques for quantifying the hydrophobicity of these products, including water vapor adsorption, contact angle measurement, and infrared spectroscopy. It explores applications of hydrophobic AEROSIL in the coatings industry, focusing on their effects on rheology, dispersion, coating properties, corrosion protection and more.