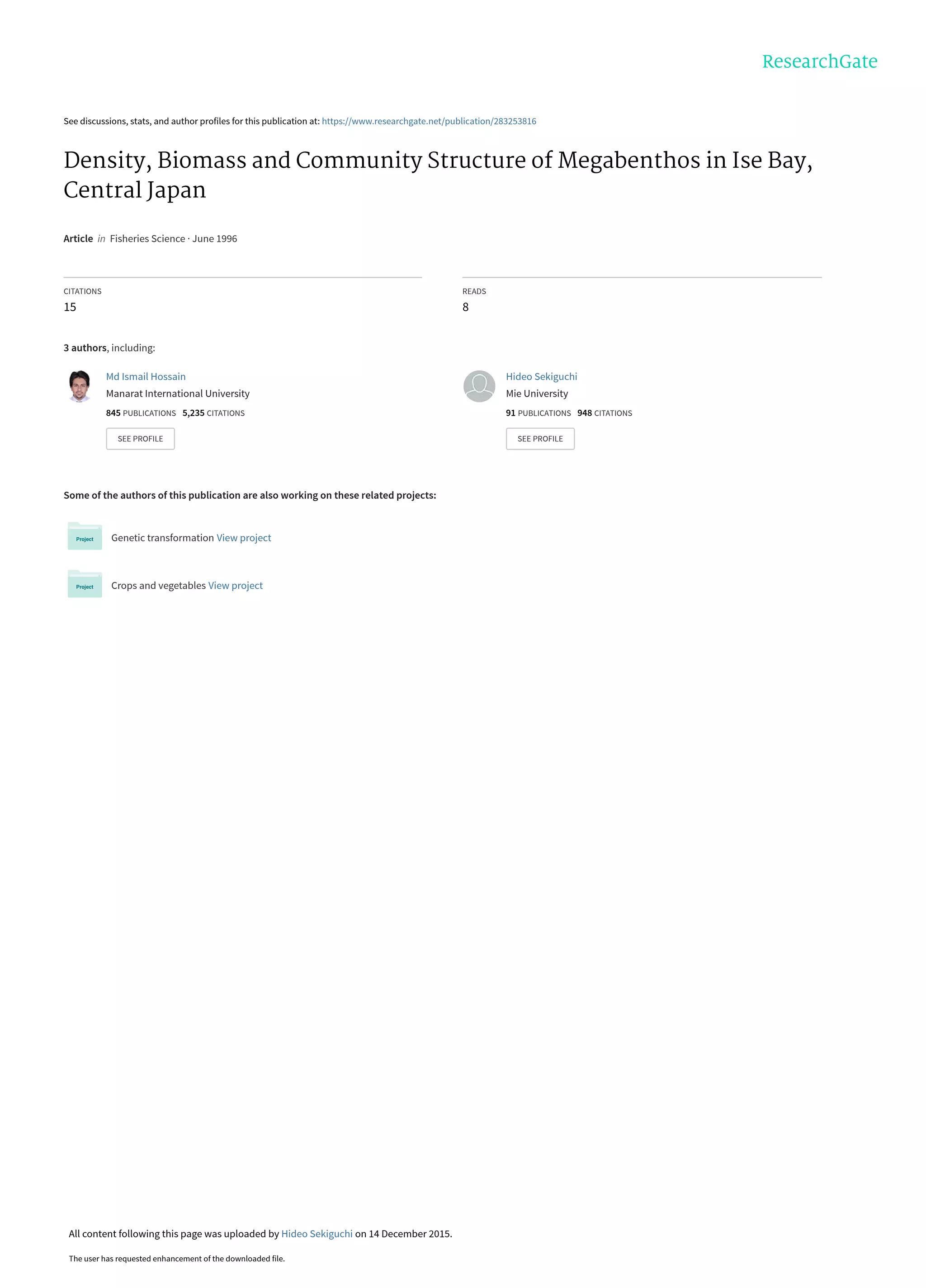
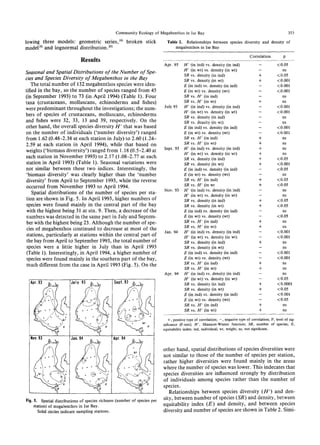
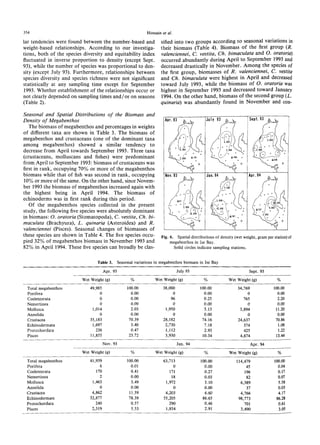
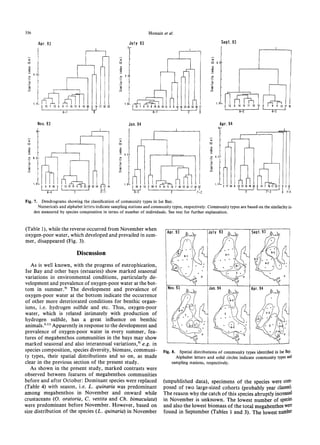
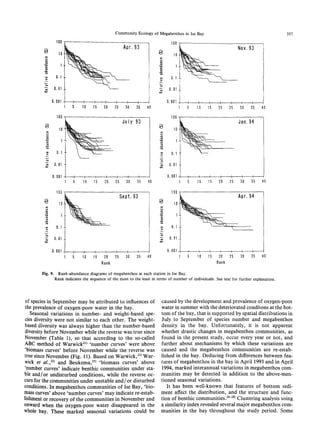
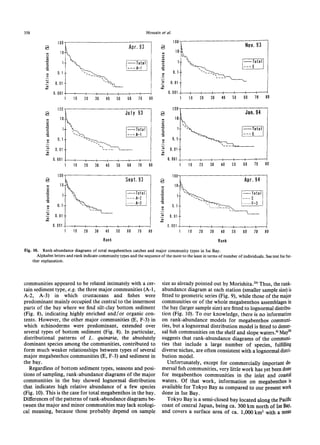
The document summarizes a study on the density, biomass, and community structure of megabenthos in Ise Bay, Japan from 1993 to 1994. Five species dominated the megabenthos communities: Oratosquilla oratoria, Carcinoplax vestita, Charybdis bimaculata, Luidia quinaria, and Repomucenus valenciennei. The community structure changed seasonally, closely related to the development and disappearance of low-oxygen water in the bay each summer. A total of 132 megabenthos species were identified, with crustaceans, mollusks, echinoderms, and fishes being the most common