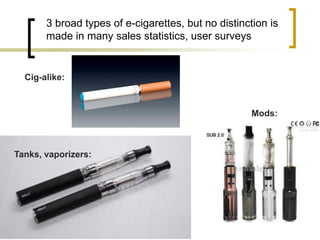
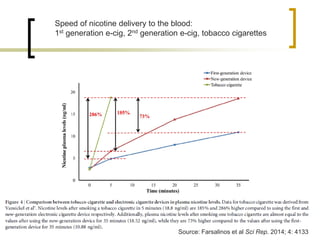
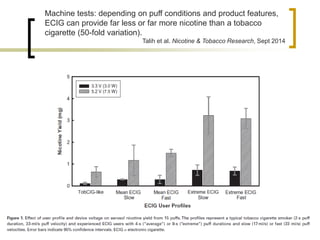
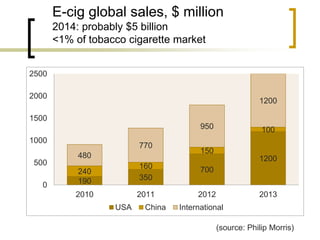
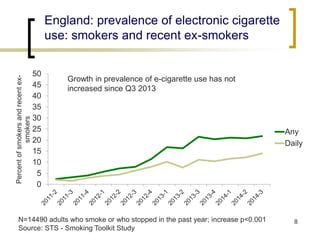
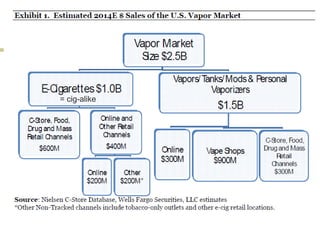
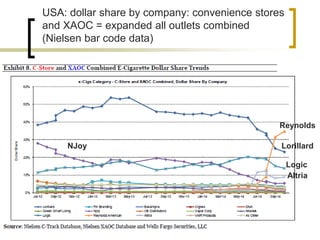
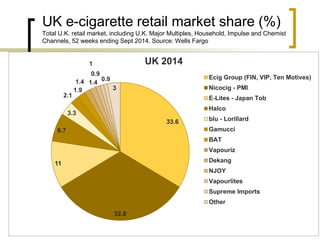
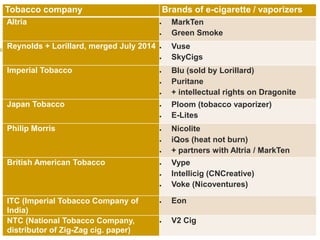
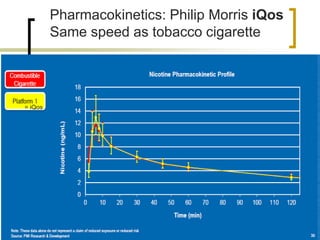
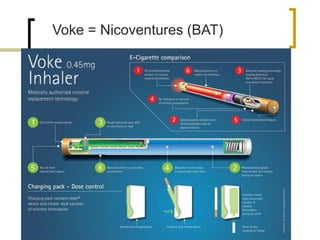
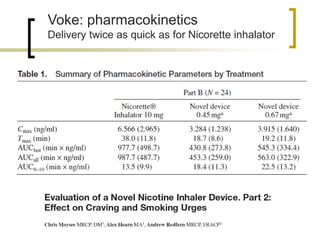
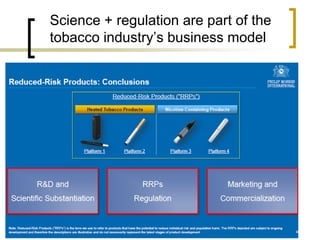
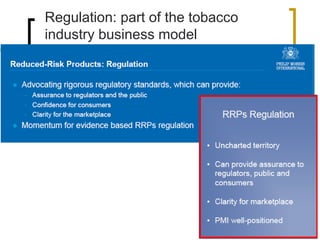
Professor Jean-François Etter discusses the evolving e-cigarette and vaporizer market. He notes that major tobacco companies like Philip Morris and British American Tobacco are now major players in the e-cigarette industry through brands like iQos and Voke. Independent research on these new products is needed, as the tobacco industry's past shows a history of deception. Frameworks for managing conflicts of interest are crucial to preserve the integrity of research and public trust. Academia must thoughtfully rethink interactions with industries producing nicotine and tobacco vaporizers.