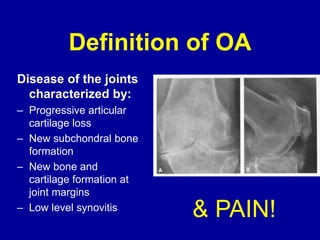
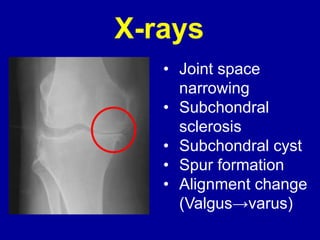
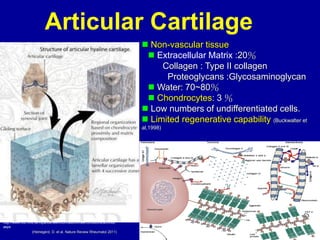
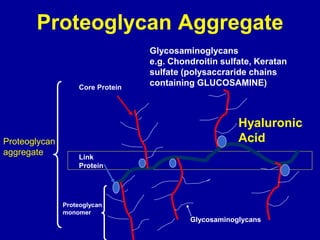
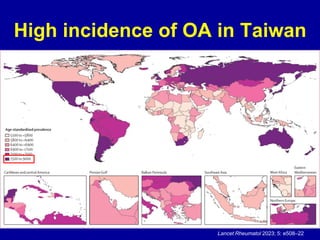
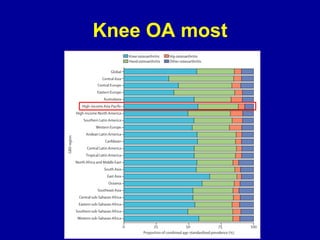
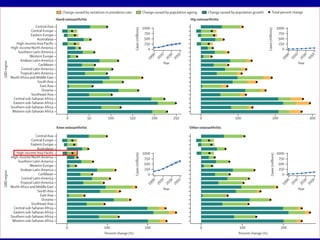
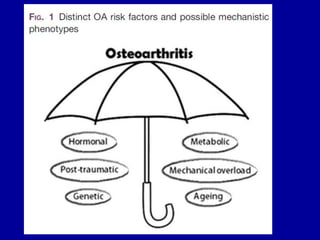
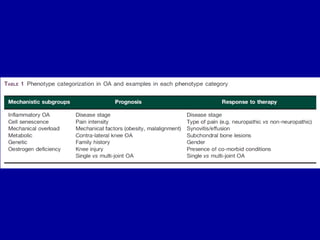
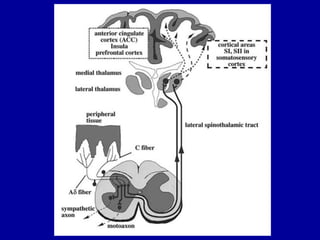
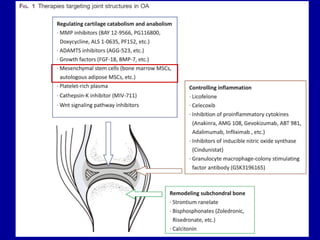
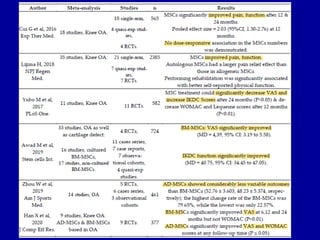
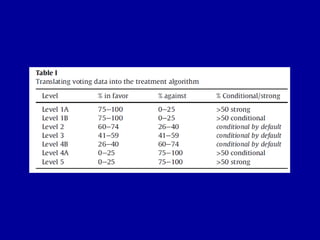
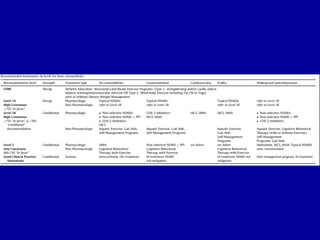
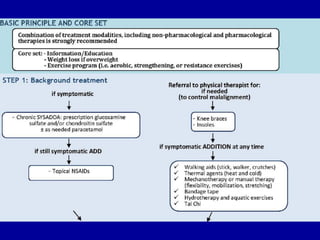
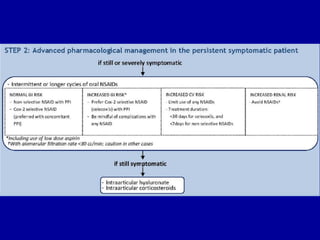
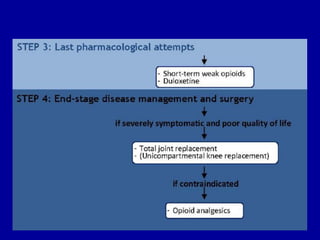
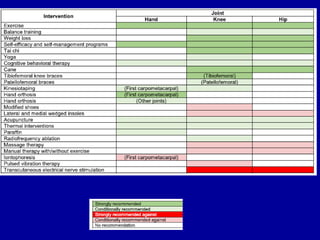
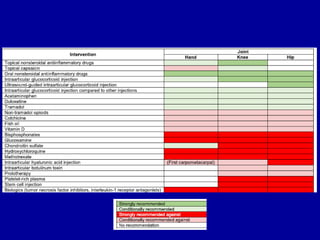
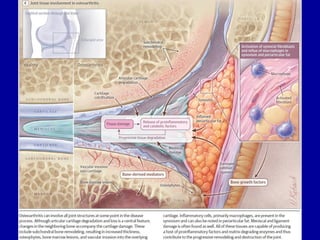
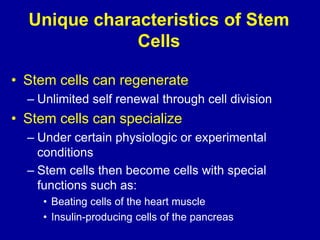
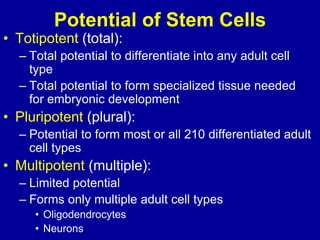
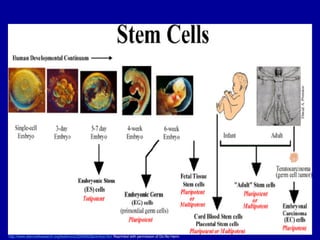
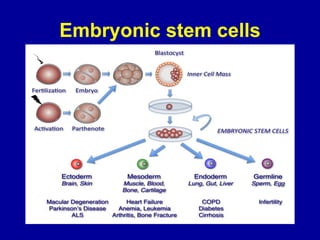
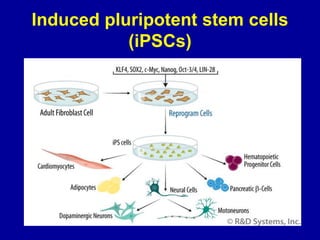
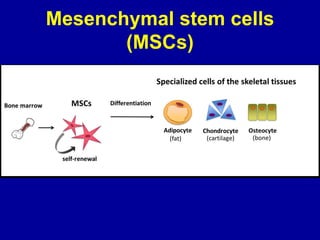
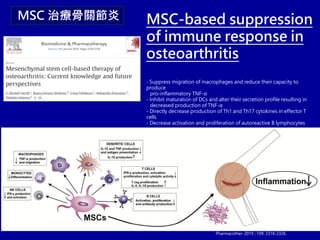
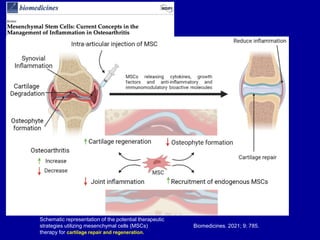
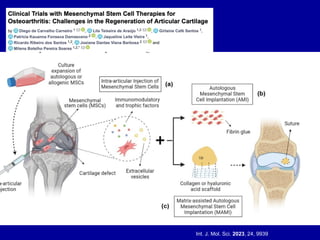
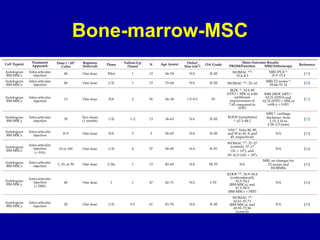
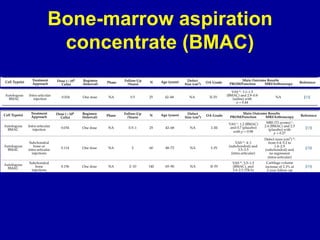
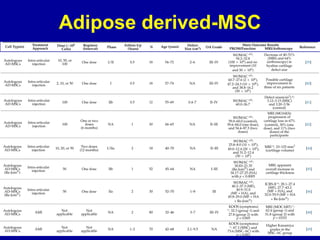
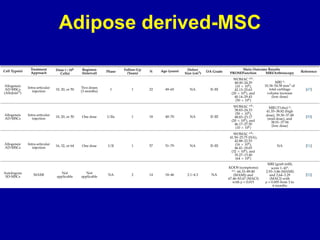
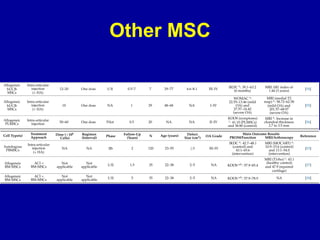
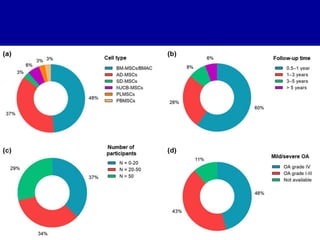
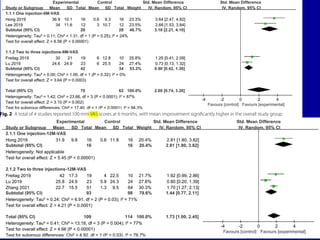
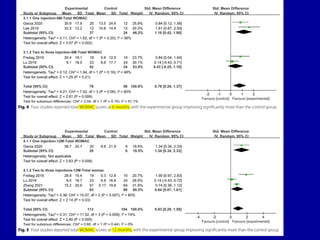
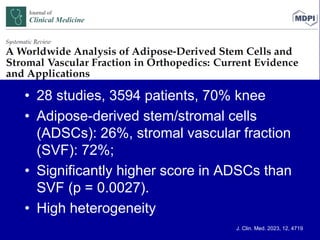
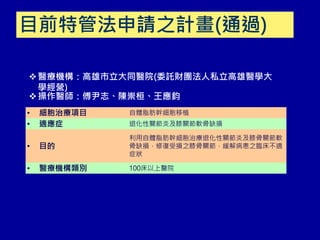
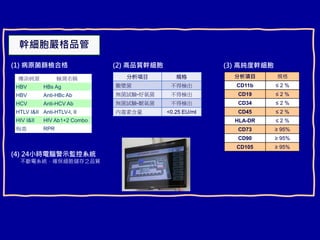
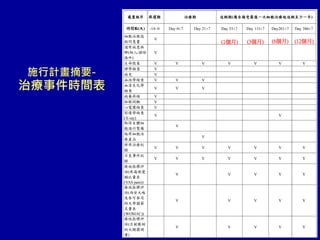
This document discusses osteoarthritis (OA) and mesenchymal stem cell (MSC) therapy for OA. It provides an overview of OA, including pathogenesis, risk factors, and current treatment approaches. It then discusses MSCs and their potential therapeutic application for OA, including how MSCs may suppress immune response and inflammation in OA joints. The document notes some safety issues with MSC therapy but also clinical studies showing benefits of MSC treatment for pain relief and functional improvement in OA patients. It describes methods of obtaining MSCs from bone marrow and adipose tissue and regulatory guidelines for preparing cell products in Taiwan.