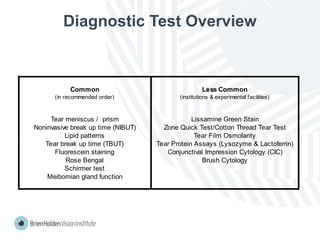
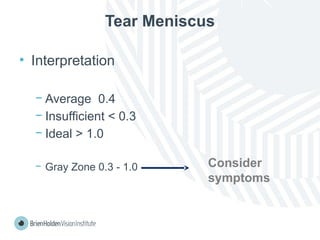
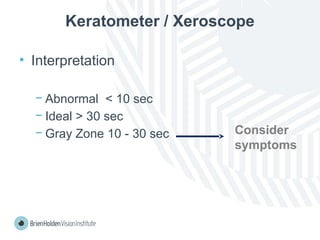
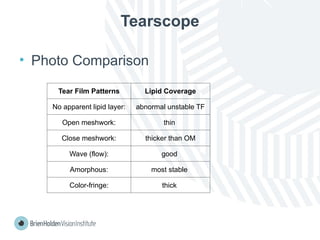
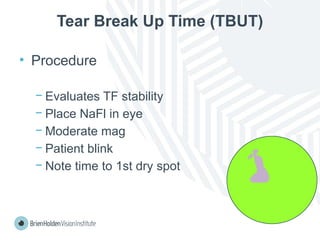
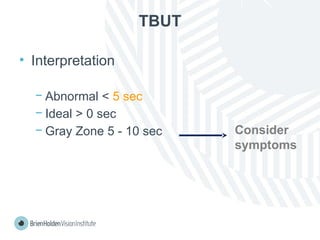
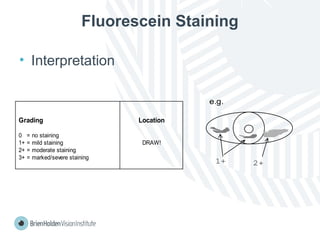
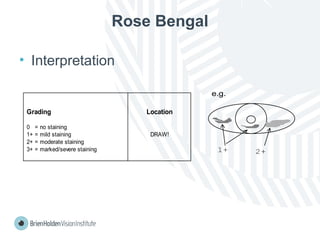
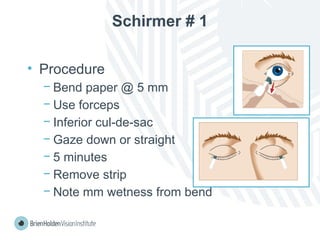
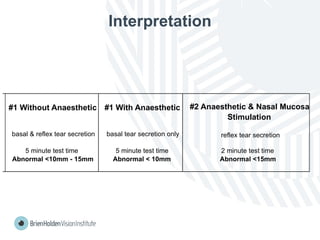
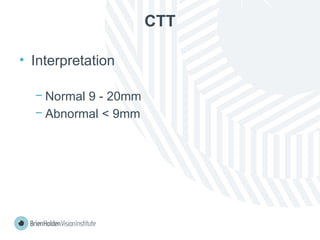
The document outlines clinical optometric procedures for diagnosing eyelid conditions, detailing various diagnostic tests and their interpretations. It emphasizes the need for qualified professional advice and provides specific procedures for tests such as tear meniscus, tear film osmolarity, and meibomian gland function. The publication is intended for general information and includes a disclaimer regarding its use and copyright.