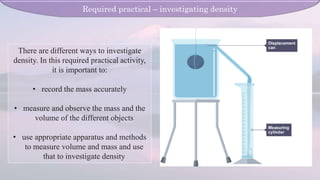
The document covers the concepts of density and pressure related to solids, liquids, and gases, including key relationships such as density = mass/volume and pressure = force/area. It details methods for measuring density through practical experiments and explains how pressure acts in fluids, including its dependence on depth and density. Additionally, it discusses atmospheric pressure and the use of barometers to measure it.