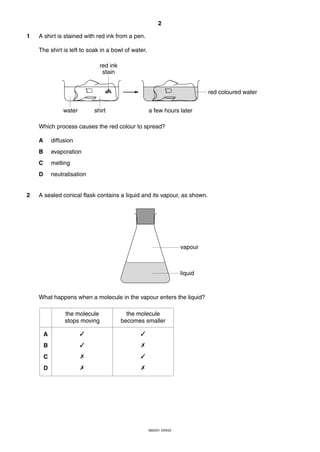
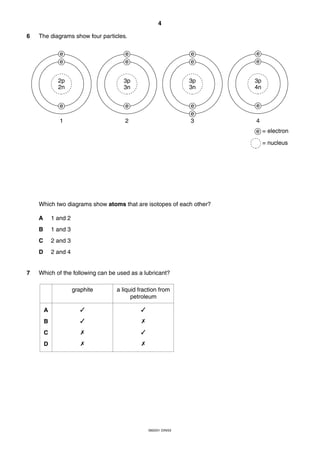
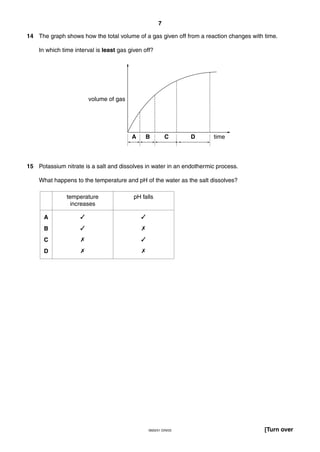
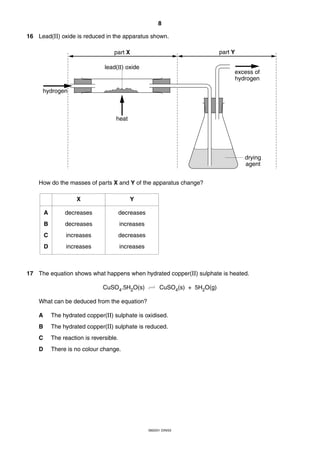
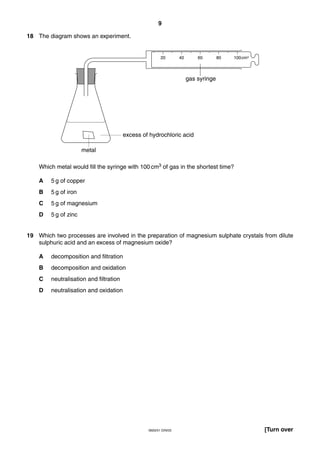
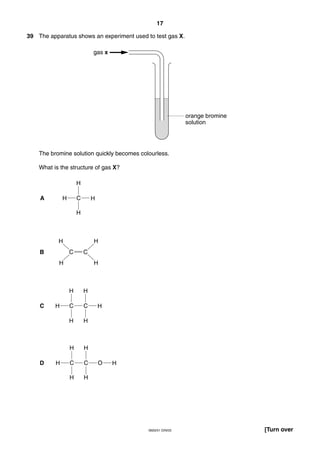
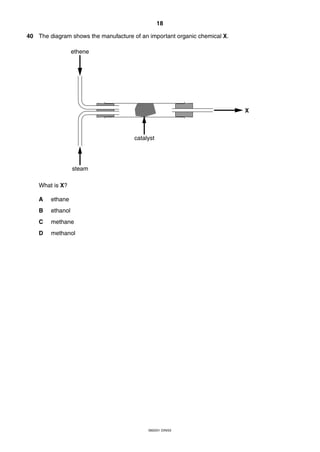
This document is a 19-page chemistry exam consisting of multiple choice questions. It includes a periodic table and instructions to answer 40 questions in 45 minutes by choosing the correct answer and recording it on an answer sheet. The exam covers topics like chemical reactions, properties of elements, acids and bases, and organic chemistry.