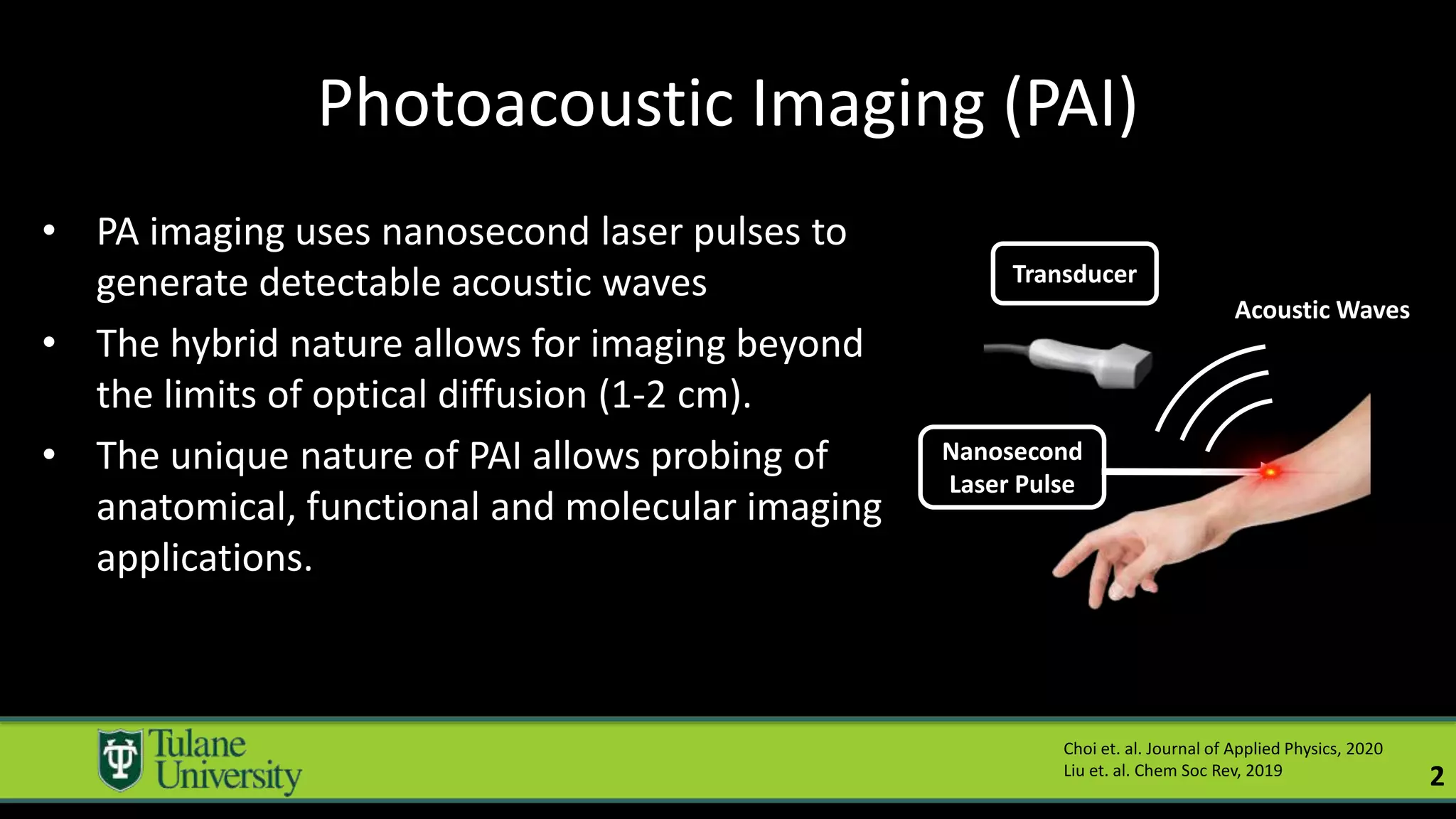
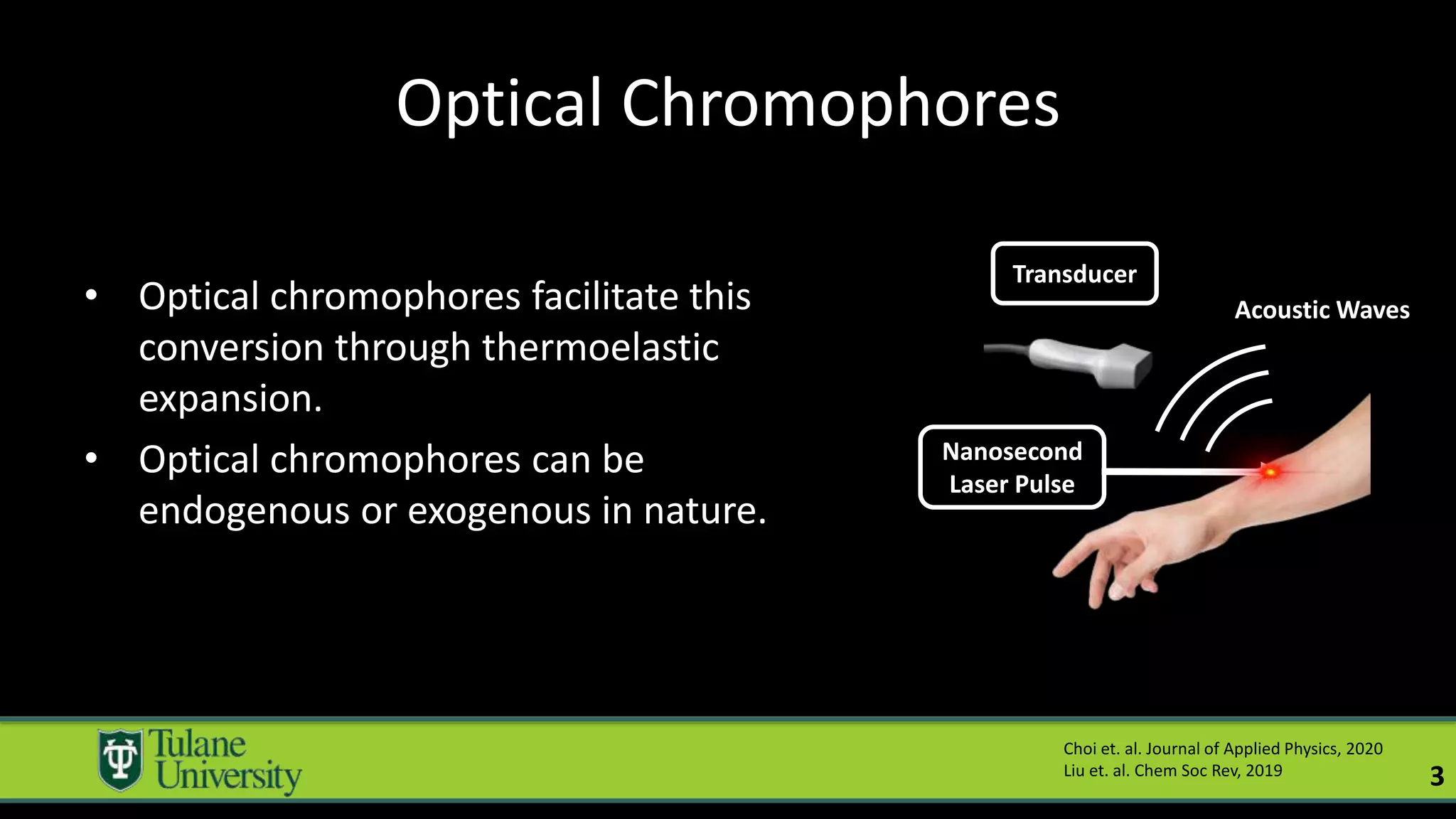
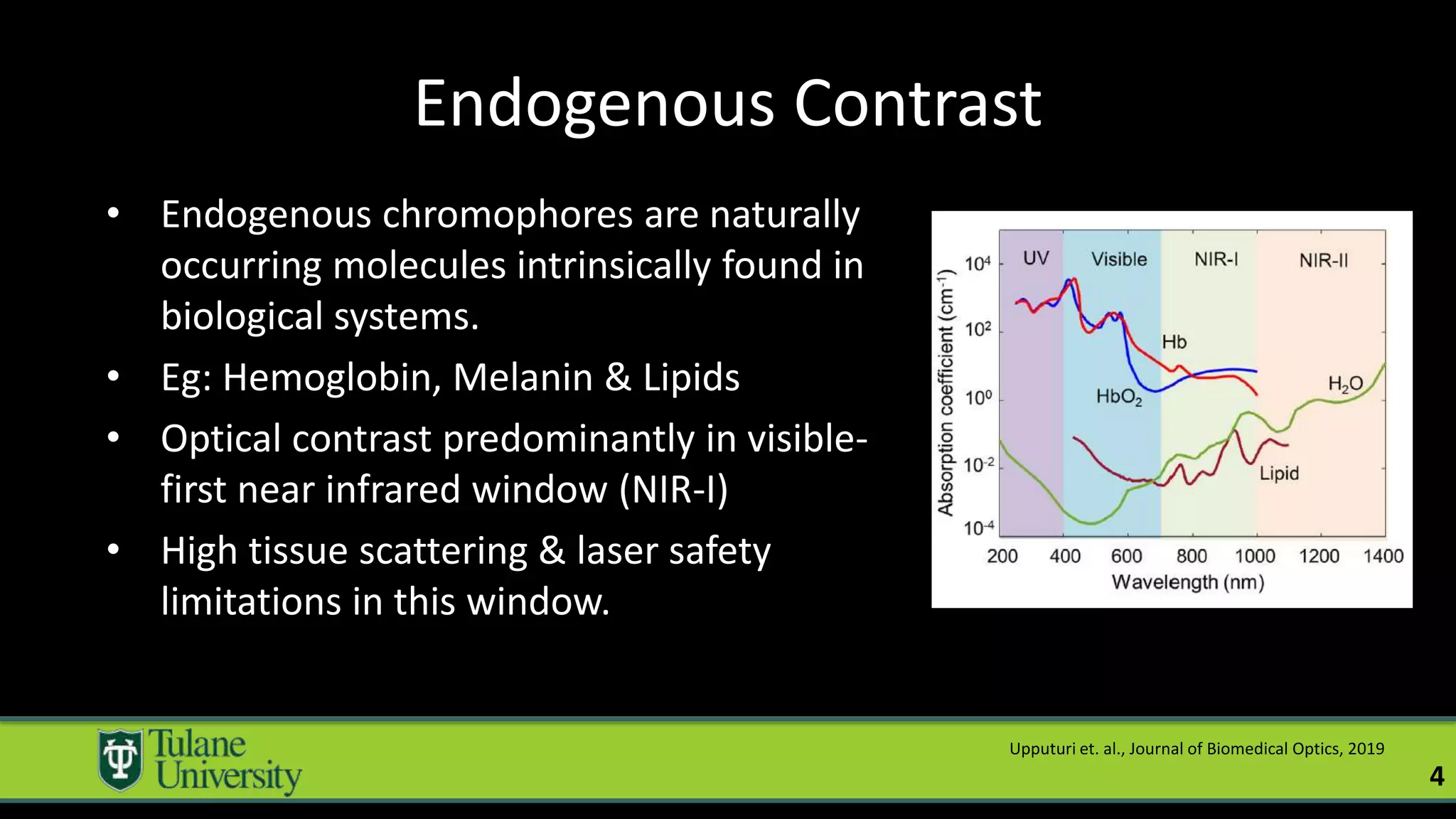
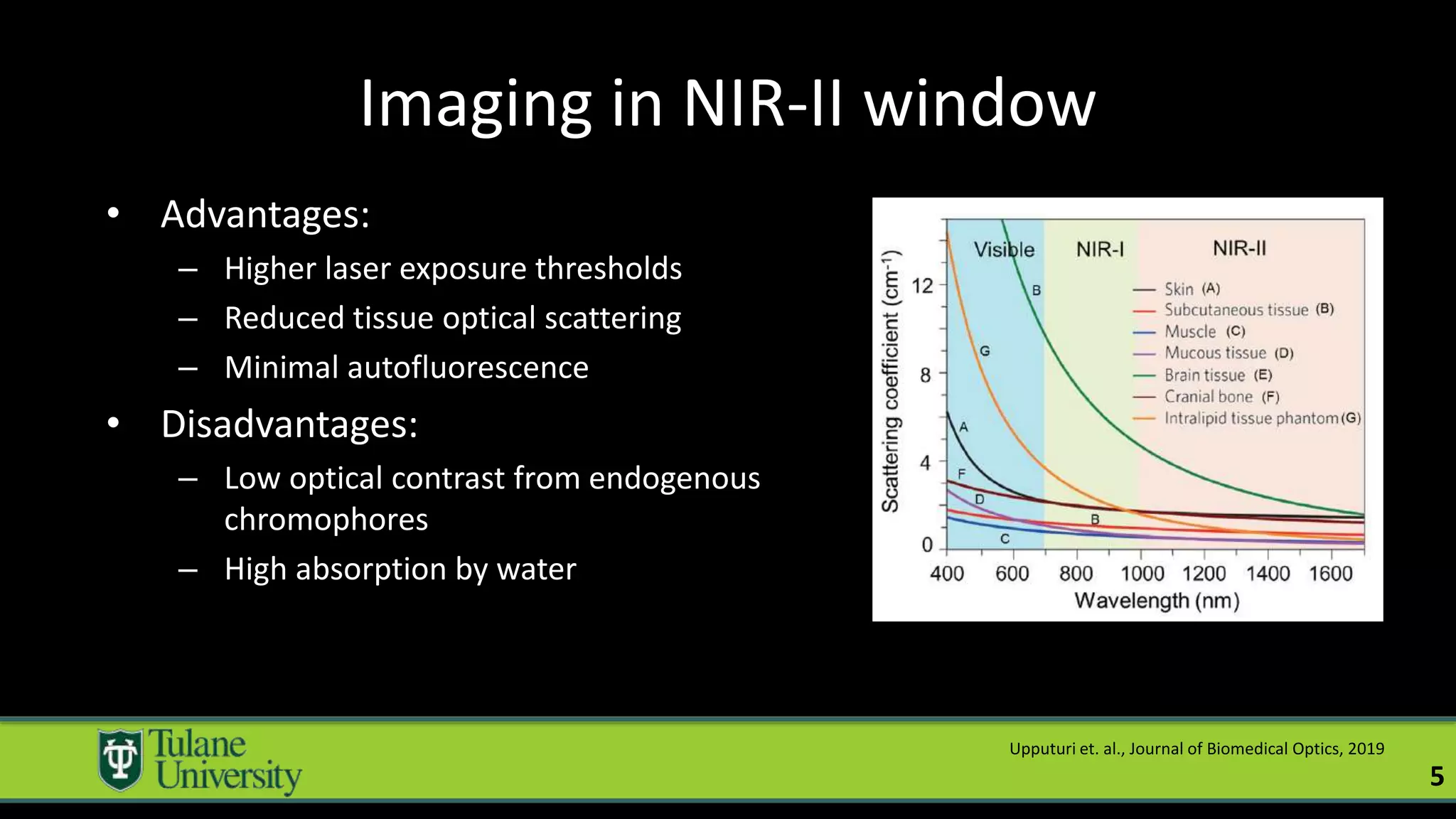
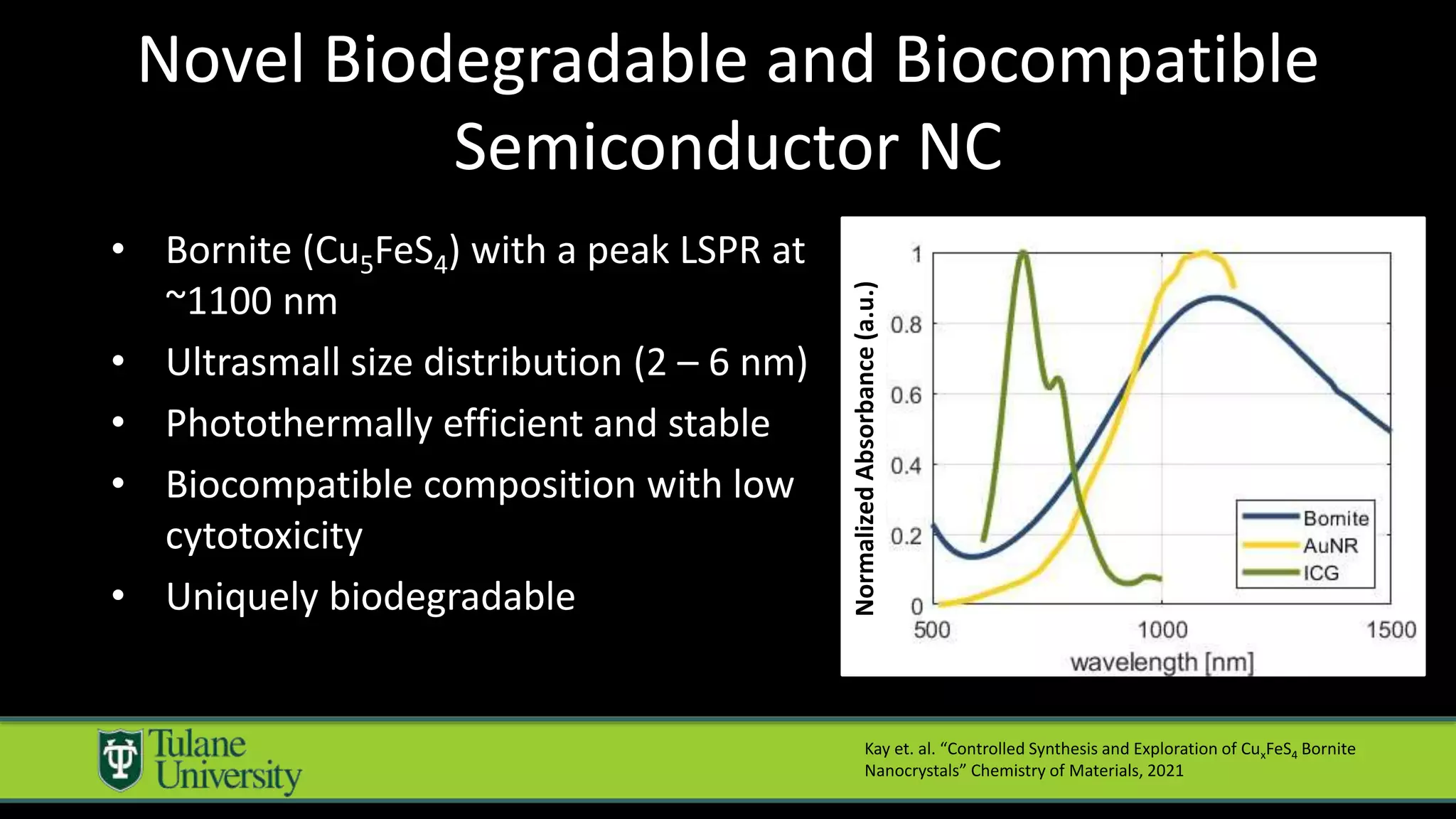
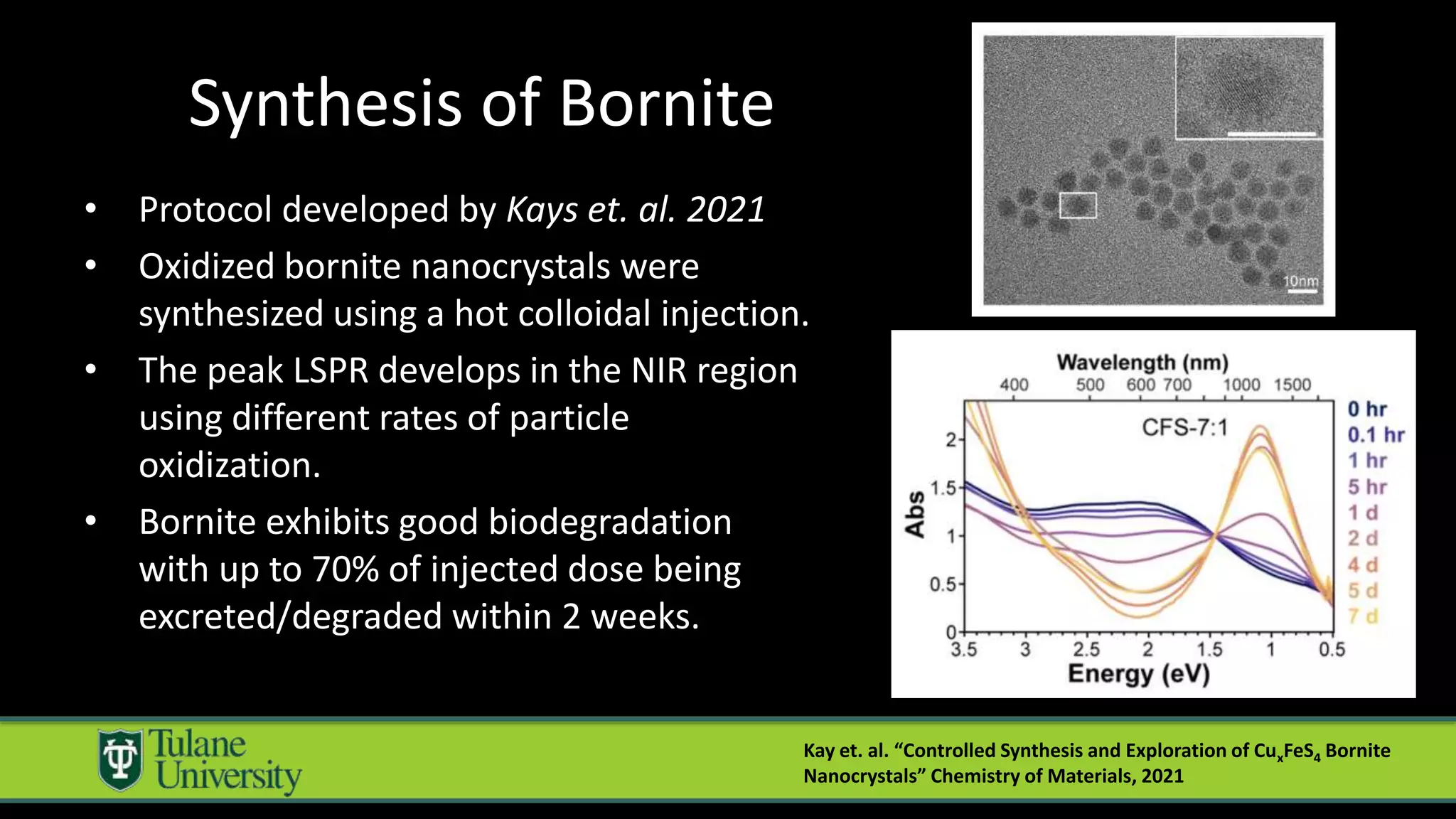
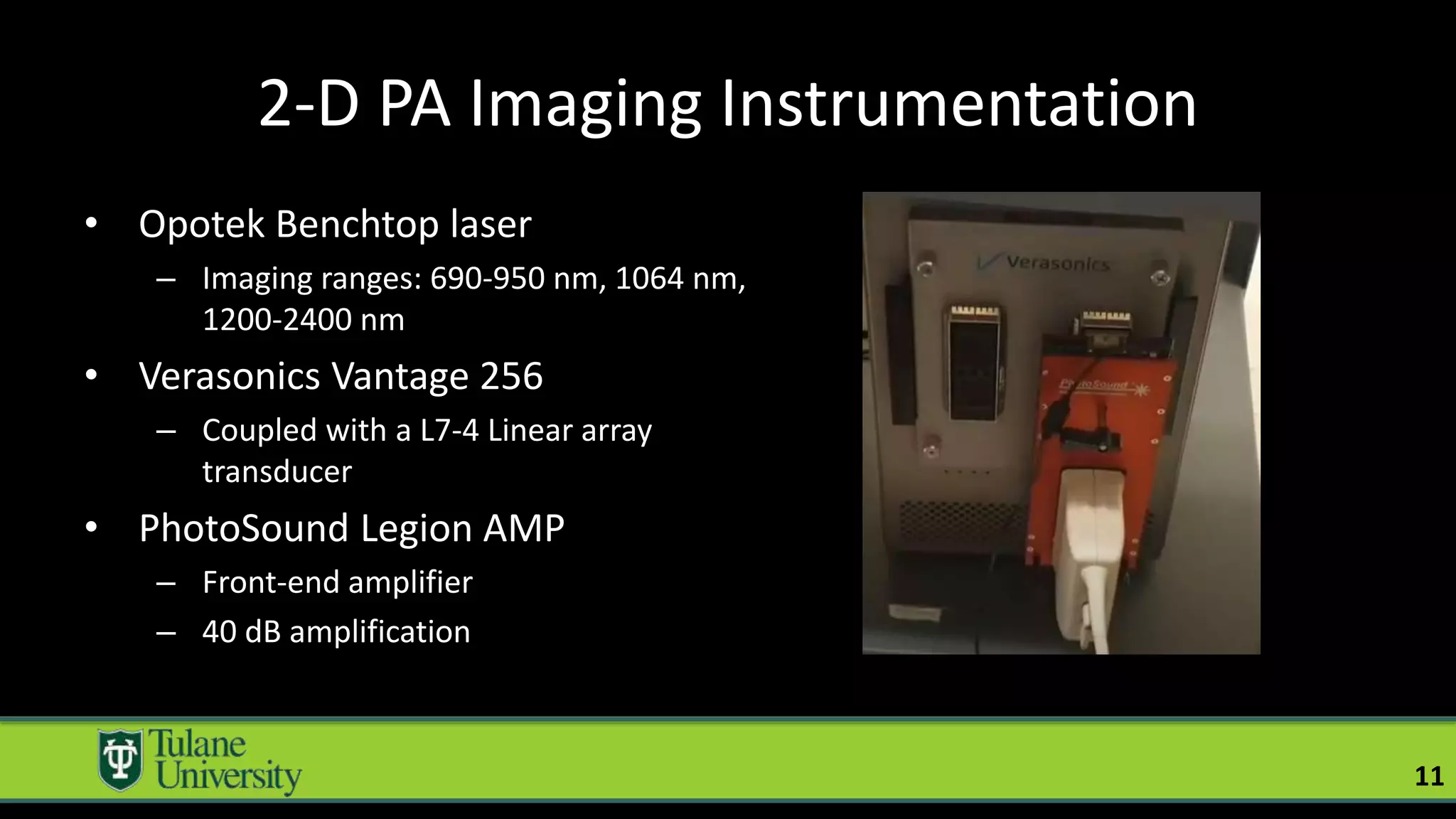
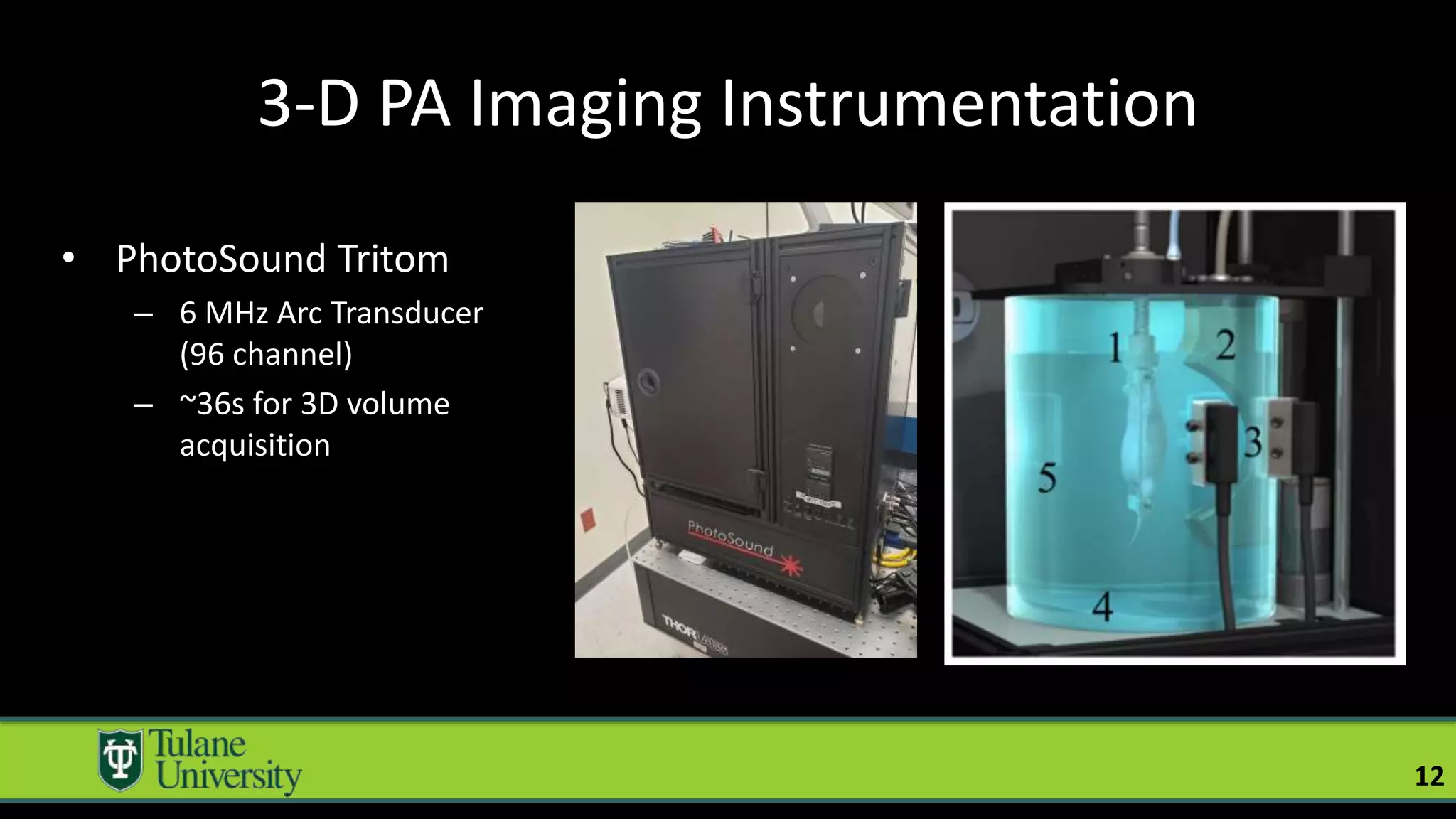
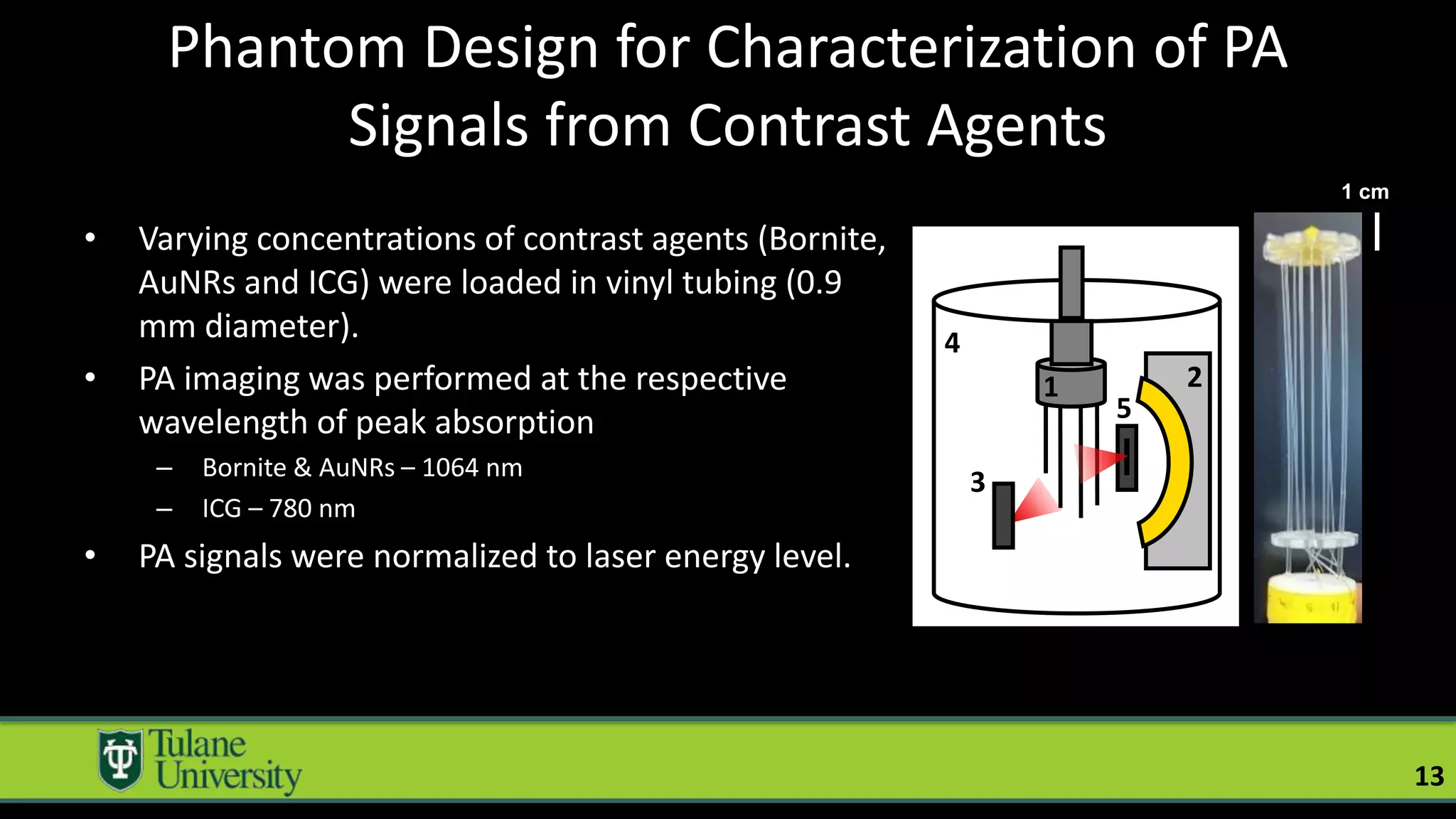
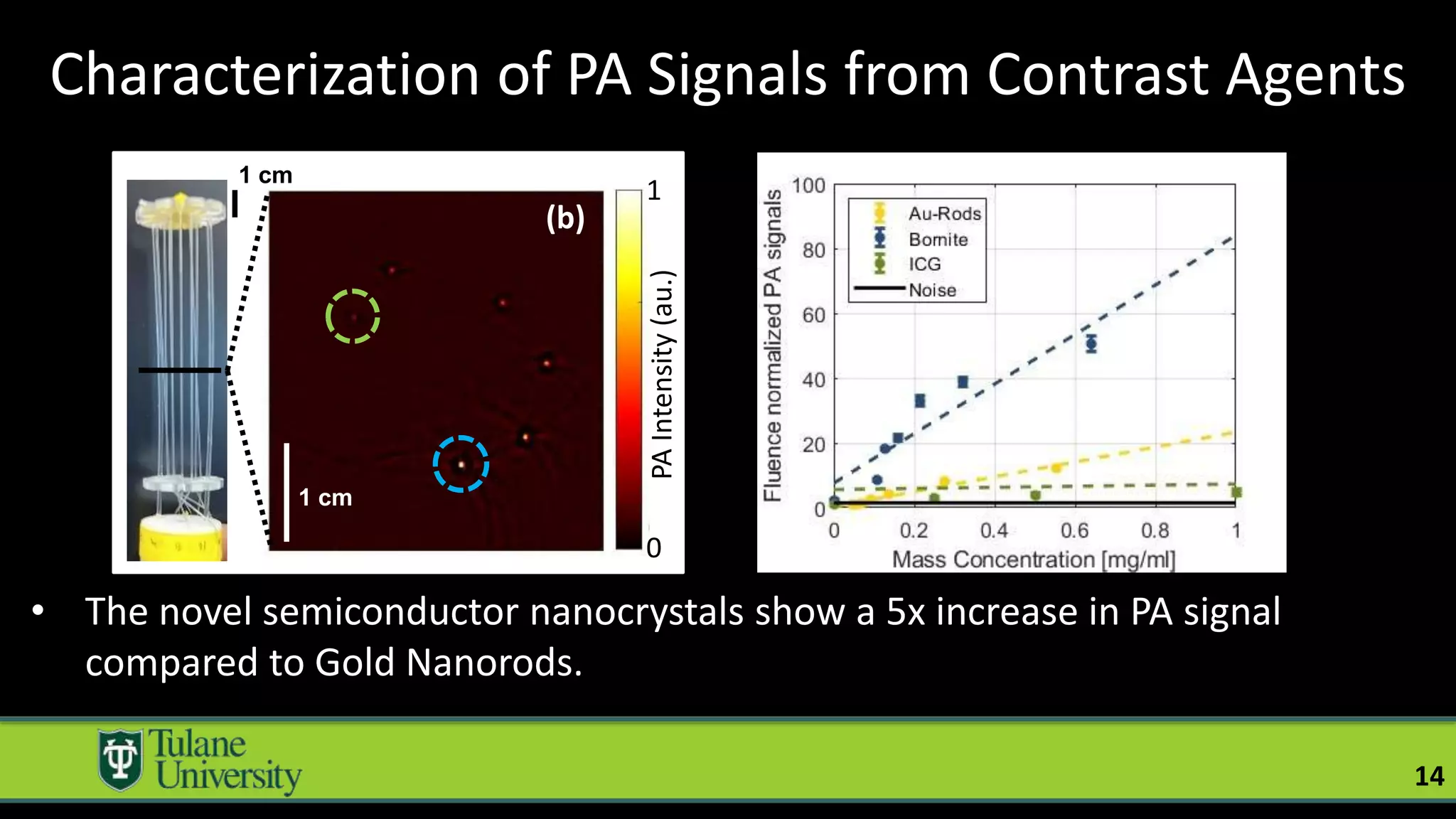
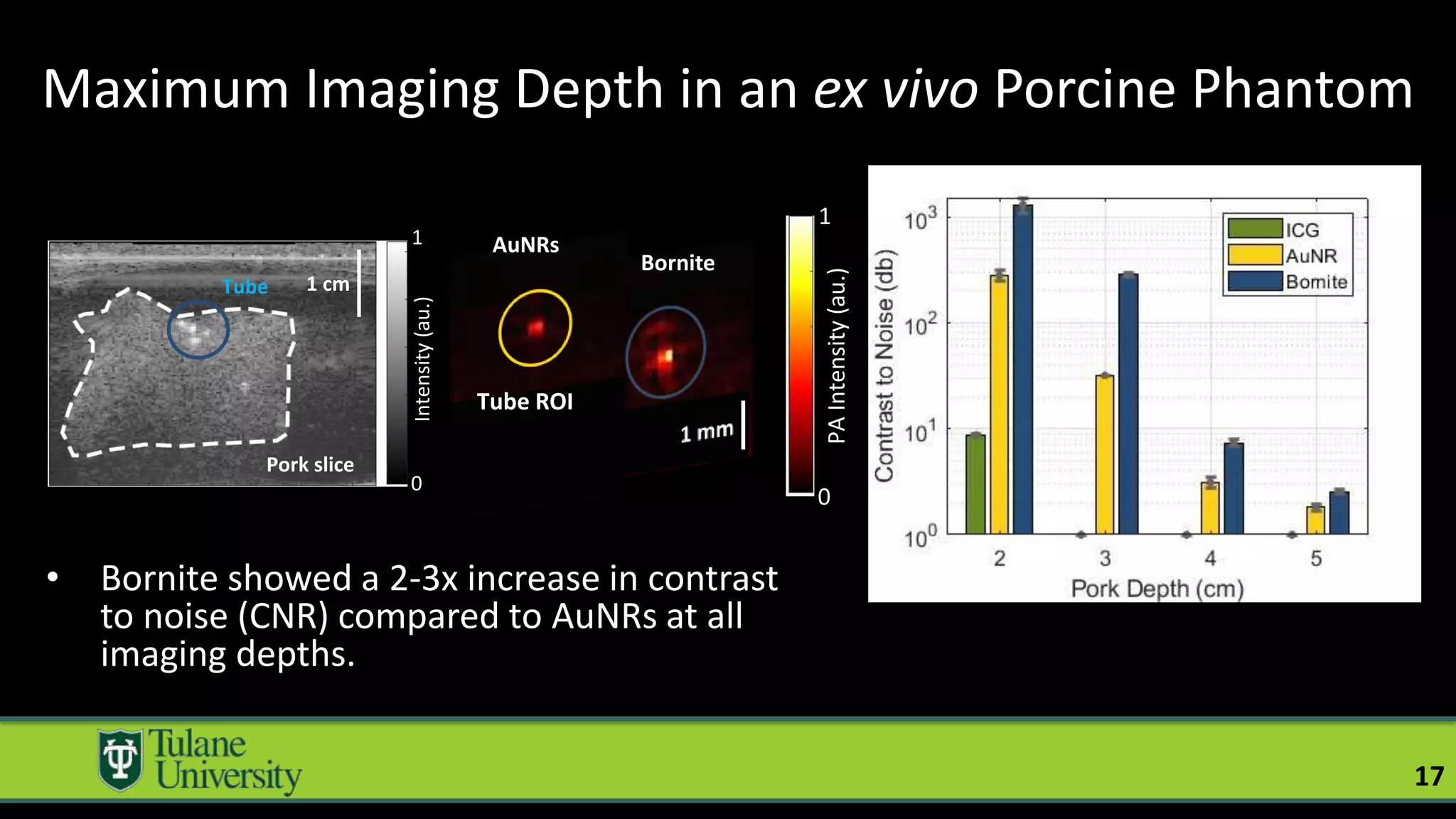
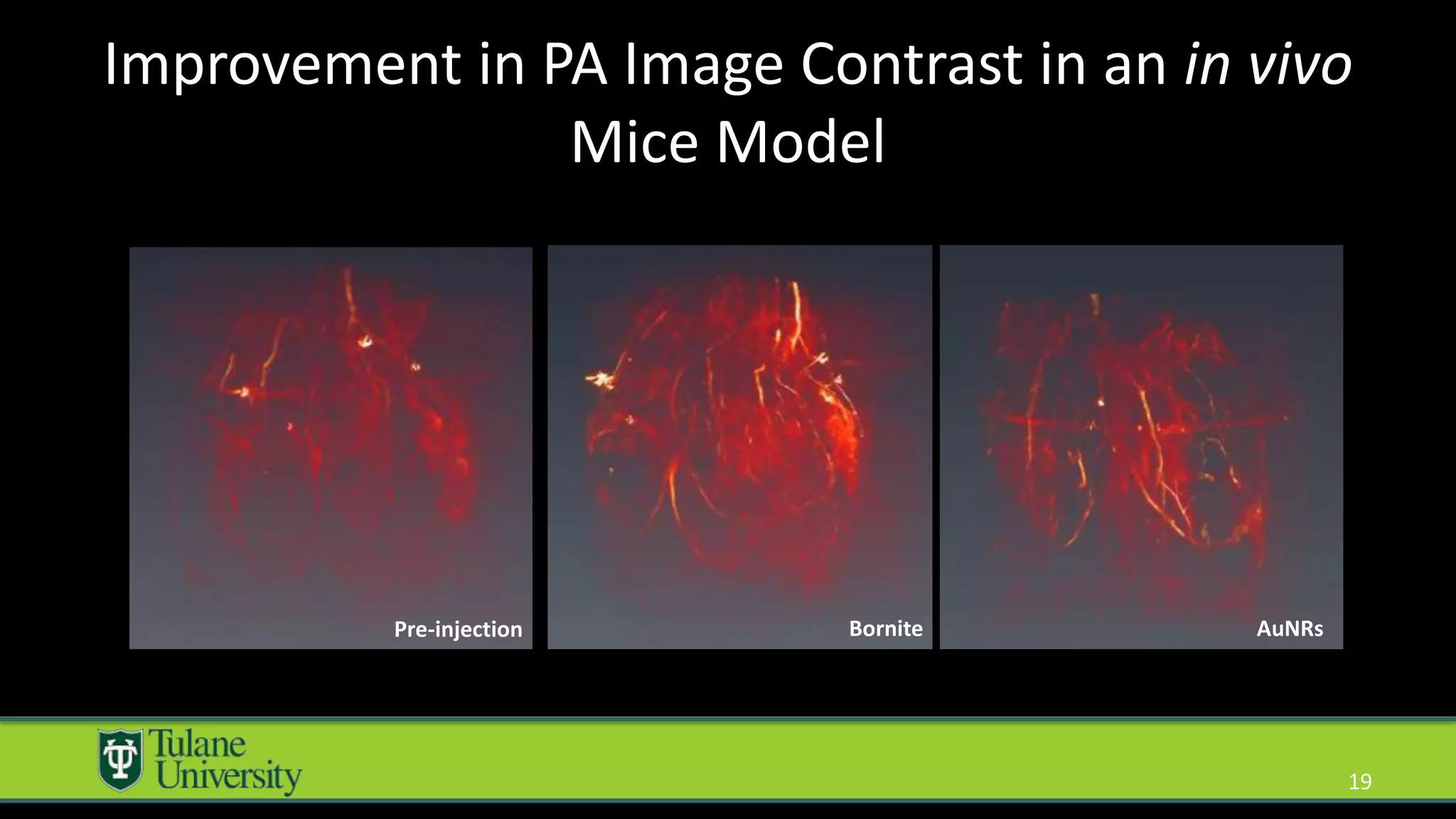
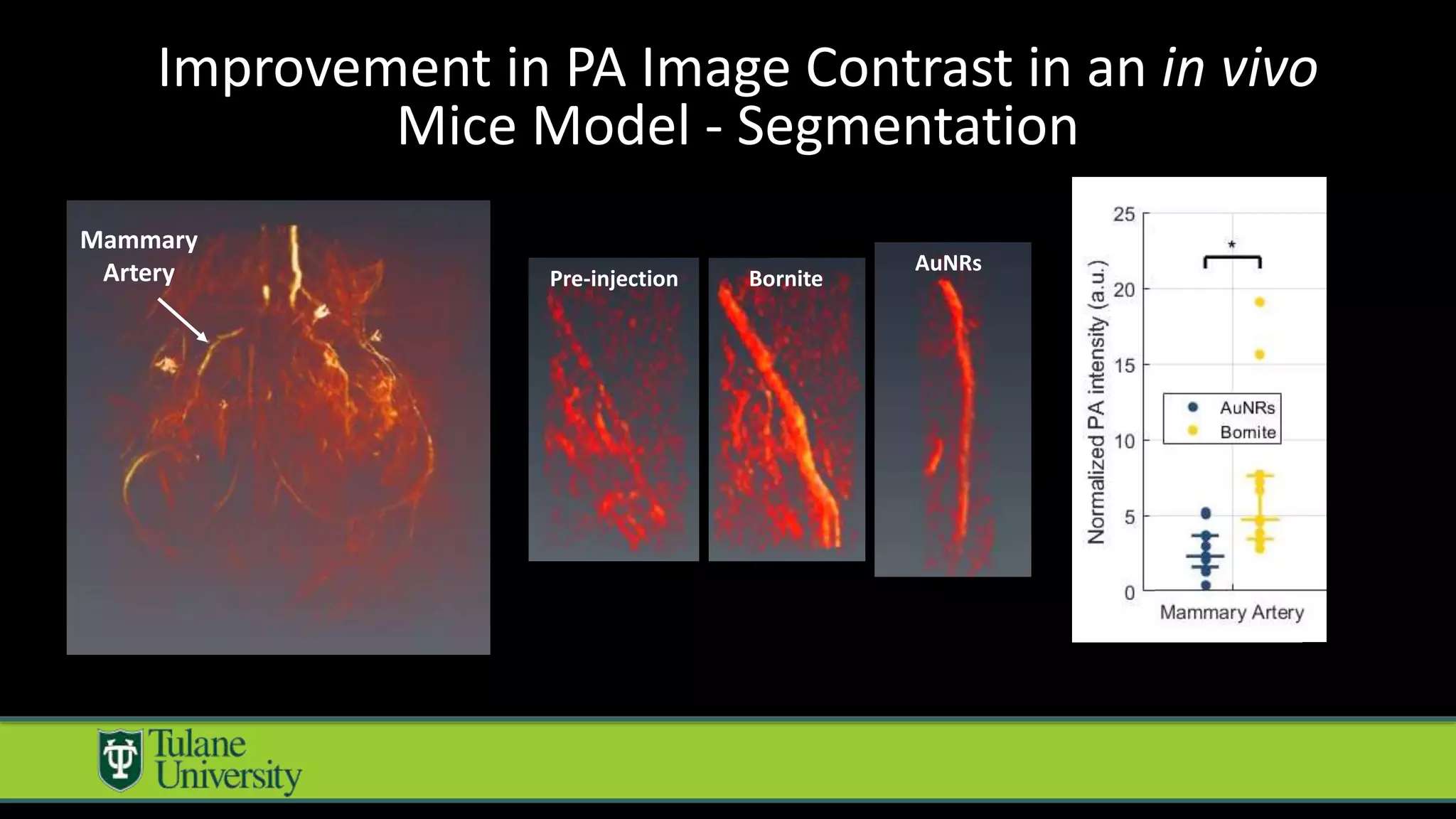
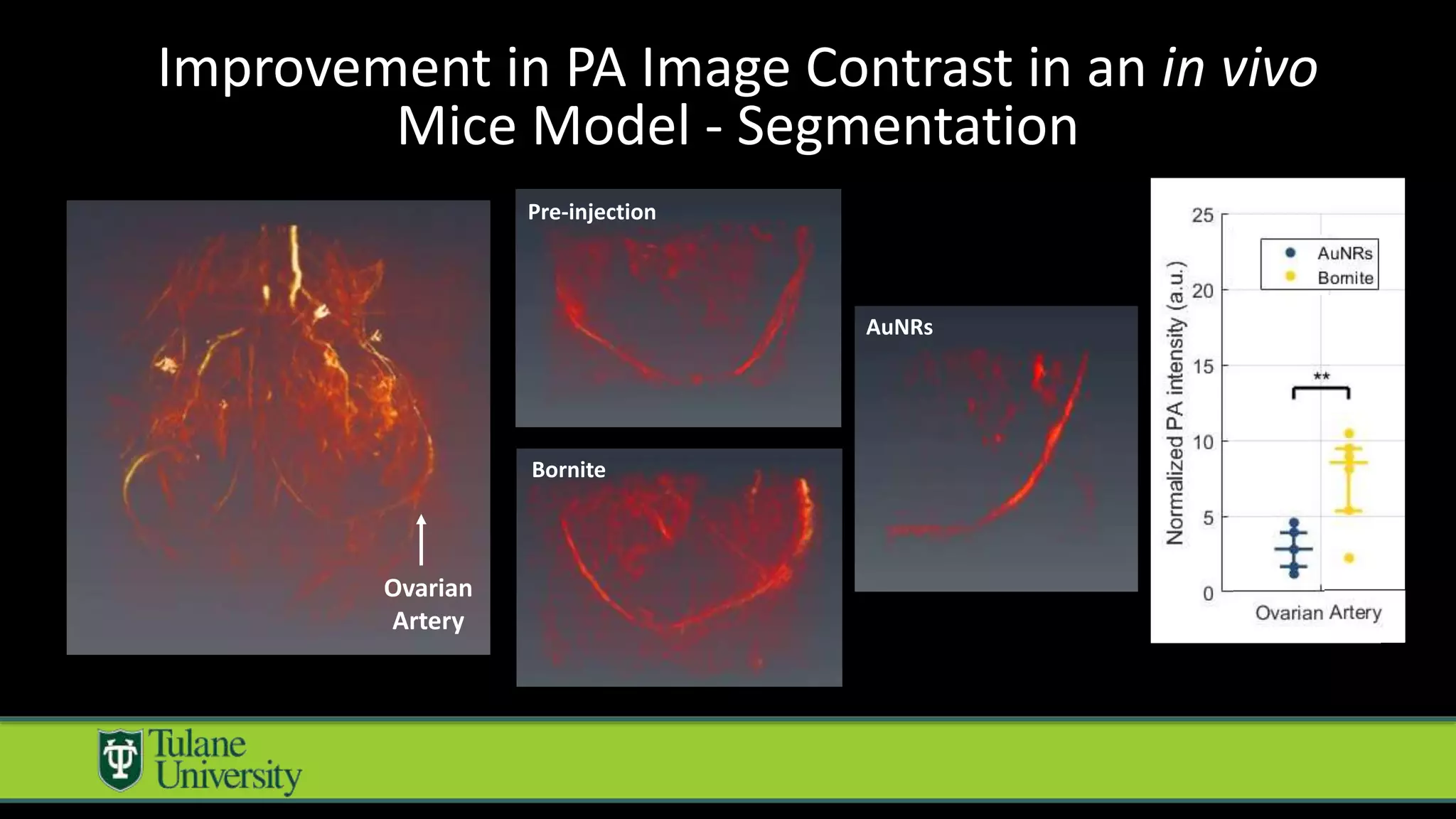
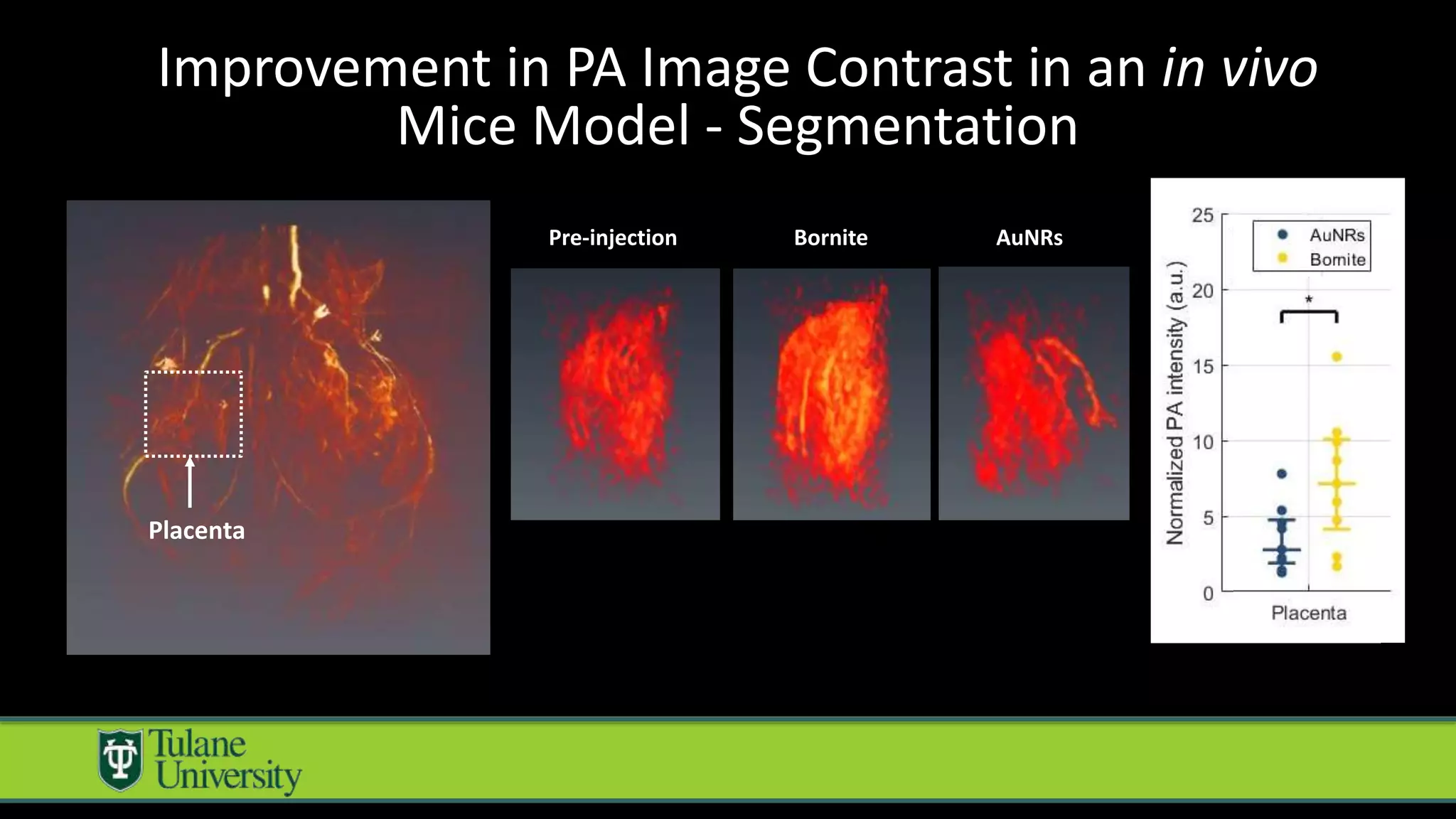
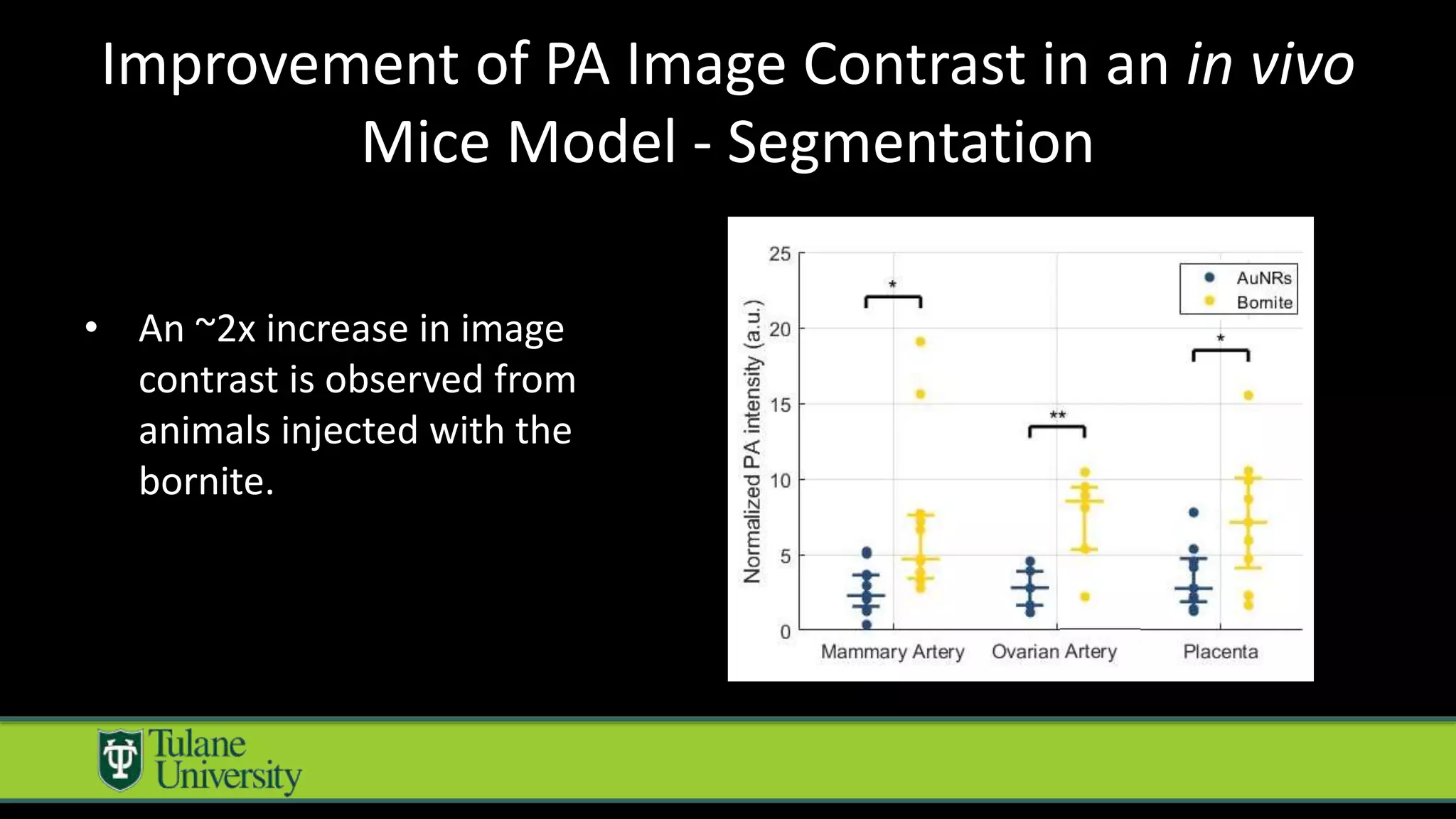
The document introduces a novel biodegradable and biocompatible semiconductor nanocrystal called bornite that could improve photoacoustic imaging contrast for deep tissue applications. Experiments show bornite generates a 5x stronger photoacoustic signal than gold nanorods and indocyanine green. It also allows 2-3x deeper imaging of up to 5cm in tissue phantoms and provides around 2x better contrast in vivo. Bornite could be a safer and more effective photoacoustic contrast agent compared to existing alternatives.