Result & discussion uv vis (exp 3) - for merge
•Download as DOCX, PDF•
0 likes•188 views
Measure the absorbance of Carmosine Standard Solution
Report
Share
Report
Share
Recommended
Recommended
This presentation reports on the development of a GC FID method to accurately quantify ethanol and IPA concentrations in two hand sanitizer samples. By using nitrogen as the carrier gas, this method is cost-effective and ensures the product compliance with CDC and USP guidelines and regulations.Determination of Ethanol and Isopropanol Content in Hand Sanitizers Using Nit...

Determination of Ethanol and Isopropanol Content in Hand Sanitizers Using Nit...Shimadzu Scientific Instruments
More Related Content
What's hot
This presentation reports on the development of a GC FID method to accurately quantify ethanol and IPA concentrations in two hand sanitizer samples. By using nitrogen as the carrier gas, this method is cost-effective and ensures the product compliance with CDC and USP guidelines and regulations.Determination of Ethanol and Isopropanol Content in Hand Sanitizers Using Nit...

Determination of Ethanol and Isopropanol Content in Hand Sanitizers Using Nit...Shimadzu Scientific Instruments
What's hot (20)
High performed liquid chromatography (HPLC) or High pressure liquid chromatog...

High performed liquid chromatography (HPLC) or High pressure liquid chromatog...
Determination of Ethanol and Isopropanol Content in Hand Sanitizers Using Nit...

Determination of Ethanol and Isopropanol Content in Hand Sanitizers Using Nit...
Micronization - Solubility Enhancement by Micronization

Micronization - Solubility Enhancement by Micronization
Similar to Result & discussion uv vis (exp 3) - for merge
Similar to Result & discussion uv vis (exp 3) - for merge (20)
a simple multi-utility miniature device used as test tube to 3D cell culture ...

a simple multi-utility miniature device used as test tube to 3D cell culture ...
Reducing the Effects of Sample Solvent on UHPLC Analyses

Reducing the Effects of Sample Solvent on UHPLC Analyses
Stability indicating method development and validation for the estimation of ...

Stability indicating method development and validation for the estimation of ...
Validated RP-HPLC Method for the Determination of Nelaribine in Bulk and Tabl...

Validated RP-HPLC Method for the Determination of Nelaribine in Bulk and Tabl...
To estimate the concentration of KMnO4 and CuSO4 solutions, colorimetrically

To estimate the concentration of KMnO4 and CuSO4 solutions, colorimetrically
Estimation of chromium (vi) by spectrophotometric method

Estimation of chromium (vi) by spectrophotometric method
Determination of Chloramphenicol in Bulk Drug and Pharmaceutical Dosage Forms...

Determination of Chloramphenicol in Bulk Drug and Pharmaceutical Dosage Forms...
Scanned with CamScanner1 STANDARIZATION OF A B.docx

Scanned with CamScanner1 STANDARIZATION OF A B.docx
SPECTROSCOPIC METHOD DEVELOPMENT FOR LOSARTON POTASSIUM IN TABLET By Ninad M...

SPECTROSCOPIC METHOD DEVELOPMENT FOR LOSARTON POTASSIUM IN TABLET By Ninad M...
Recently uploaded
From customer value engagements to hands-on production support, our Services span across every stage of our customers digital transformation journey, to help ensure that every customer is successful in their adoption of our solutions.
• Implementation, Upgrade, Migration, and Maintenance Services
• On-Premises and On-Cloud
• COTS Training Services; On-Site and Virtual
• Software Support Services; Legacy and 3DEXPERIENCE
• Value Engagement & Blueprinting
• Specialized Consulting and Support Services
• Customized Training Services
• Automation and Configuration Services
• Technical Resource Augmentation Services
• Project Management
• Know-how Training (mentoring) and Resource AugmentationNavigating Complexity: The Role of Trusted Partners and VIAS3D in Dassault Sy...

Navigating Complexity: The Role of Trusted Partners and VIAS3D in Dassault Sy...Arindam Chakraborty, Ph.D., P.E. (CA, TX)
Recently uploaded (20)
HAND TOOLS USED AT ELECTRONICS WORK PRESENTED BY KOUSTAV SARKAR

HAND TOOLS USED AT ELECTRONICS WORK PRESENTED BY KOUSTAV SARKAR
Navigating Complexity: The Role of Trusted Partners and VIAS3D in Dassault Sy...

Navigating Complexity: The Role of Trusted Partners and VIAS3D in Dassault Sy...
PE 459 LECTURE 2- natural gas basic concepts and properties

PE 459 LECTURE 2- natural gas basic concepts and properties
Block diagram reduction techniques in control systems.ppt

Block diagram reduction techniques in control systems.ppt
Bhubaneswar🌹Call Girls Bhubaneswar ❤Komal 9777949614 💟 Full Trusted CALL GIRL...

Bhubaneswar🌹Call Girls Bhubaneswar ❤Komal 9777949614 💟 Full Trusted CALL GIRL...
NO1 Top No1 Amil Baba In Azad Kashmir, Kashmir Black Magic Specialist Expert ...

NO1 Top No1 Amil Baba In Azad Kashmir, Kashmir Black Magic Specialist Expert ...
Verification of thevenin's theorem for BEEE Lab (1).pptx

Verification of thevenin's theorem for BEEE Lab (1).pptx
Result & discussion uv vis (exp 3) - for merge
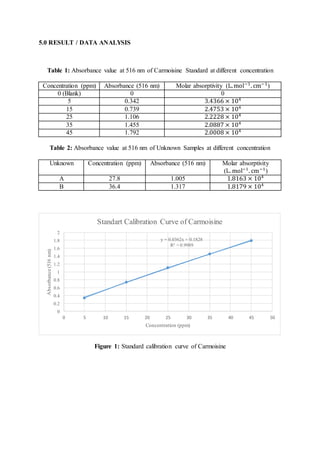
- 1. 5.0 RESULT / DATA ANALYSIS Table 1: Absorbance value at 516 nm of Carmoisine Standard at different concentration Concentration (ppm) Absorbance (516 nm) Molar absorptivity (L.mol−1 . cm−1 ) 0 (Blank) 0 0 5 0.342 3.4366 × 104 15 0.739 2.4753 × 104 25 1.106 2.2228 × 104 35 1.455 2.0887 × 104 45 1.792 2.0008 × 104 Table 2: Absorbance value at 516 nm of Unknown Samples at different concentration Unknown Concentration (ppm) Absorbance (516 nm) Molar absorptivity (L. mol−1 . cm−1 ) A 27.8 1.005 1.8163 × 104 B 36.4 1.317 1.8179 × 104 Figure 1: Standard calibration curve of Carmoisine y = 0.0362x + 0.1828 R² = 0.9989 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1.8 2 0 5 10 15 20 25 30 35 40 45 50 Absorbance(516nm) Concentration (ppm) Standart Calibration Curve of Carmoisine
- 2. 6.0 DISCUSSION The purpose of this experiment were to determine Lambda Max for Carmoisine sample (wavelength scan). To determine ɛ the molar absorptivity of Carmoisine and to prepare a serial dilution and generate a standard calibration graph for sample quantitation (photometric scan). The Carmoisine standard solution samples were run by using Uv-Vis Spectrophotometer. Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared (NIR)) ranges. UV-Vis spectrophotometer is used in the quantitative determination of concentrations of the absorber in the solutions of transition metal ions and highly conjugated organic compounds (IIT Kanpur, 2012). Besides, UV spectrophotometer principle follows the Beer-Lambert Law. This law states that whenever a beam of monochromatic light is passed through a solution with an absorbing substance, the decreasing rate of the radiation intensity along with the thickness of the absorbing solution is actually proportional to the concentration of the solution and the incident radiation (Choudhary, A. 2017). The experiment was started with 100 ppm of stock solution of Carmoisine was prepared to dilute the stock solution into different concentration which were 5 ppm, 15 ppm, 25 ppm, 35 ppm and 45 ppm with distilled water into 50 ml volumetric flask. The volume needed to prepare the serial dilution from 100 ppm of stock solution were 2.5 ml, 7.5 ml, 12.5 ml, 17.5 ml and 22.5 ml respectively. The volume of serial dilution were calculated by using the formula M1V1 = M2V2. Moreover, the unknown sample was prepared with the same method as serial dilution but the volume of stock solution must be between 2.5 ml to 22.5 ml. After setting up the UV-Vis, the blank solution was placed into instrument and baseline was click. After the blank solution became zero, the standard solution of 45 ppm was placed to another cuvette. The maximum wavelength was recorded at 516 nm. The maximum wavelength was the wavelength with the higher absorbance. For the photometric scan, the cuvette was filled with standard solution 5 ppm to 45 ppm to determine the absorbance and prepared for the standard calibration curve.
- 3. According to the result obtained, the absorbance reading showed for the standard solution 5 ppm, 15 ppm, 25 ppm, 35 ppm and 45 ppm were 0.342, 0.739, 1.106, 1.455 and 1.792 respectively. Based on graph plotted, the absorbance (516 nm) was directly proportional to the concentration (ppm). Because of when the concentration was higher, more radiation is absorbed and the absorbance also become higher. In addition, for the unknown samples the absorbance obtained were 1.005 and 1.317. The concentration of unknown samples have found by using Beer’s Lambert law formula A = εbc with the slope obtained was 0.0362 which indicates the molar absorptivity of Carmoisine. Thus, the concentration of unknown samples were 27.8 ppm and 36.4 ppm. Furthermore, the graph showed the R2 value that is a statistical measure of how close the data are to the fitted regression line (Ogee, A. 2013). The correlation coefficient varies about +1 to -1. The graph showed the correlation coefficient was 0.9989 which the R2 value was nearest to the +1 which indicates the accurate of the result. As conclusion, there are several possibilities precaution that should be considered. Firstly, make sure use the pipette to measure the volume of Carmoisine to get the accuracy. Secondly, make sure wipe and do not touched the clean sides of cuvette and carrying out the set-up procedure in the correct order. REFERENCES 1. Ogee, A. (2013). Regression Analysis: How Do I Interpret R-squared and Assess the Goodness-of-Fit?. Retrieved from https://blog.minitab.com/blog/adventures- in- statistics-2/regression-analysis-how-do-i-interpret-r-squared-and-assess-the-goodness- of-fit. 2. Wenzel, T. (2019). Beer’s Law. Retrieved from https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Module s_(Analytical_Chemistry)/Analytical_Sciences_Digital_Library/Active_Learning/In_ Class_Activities. 3. IIT Kanpur. (2012). UV-Vis Spectrophotometer. Retrieved from https://www.iitk.ac.in/dordold/index.php?option=com_content&view=category&layo ut=blog&id=219&Itemid=238. 4.