1) A patient with familial hypercholesterolemia (FH) on PCSK9 inhibitors described facing step therapy requirements and high copays to access the drug, with an initial $1000 copay and $400 monthly copay.
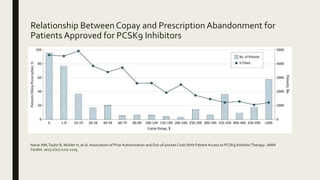
2) A study found that only 30.9% of patients prescribed PCSK9 inhibitors ever received the therapy due to prior authorization denials and high copays, with prescription abandonment rates over 75% for copays over $350.
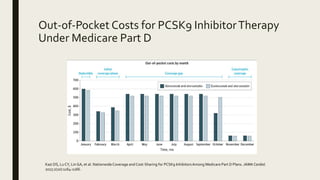
3) Medicare Part D plans have annual out-of-pocket costs for PCSK9 inhibitors and statins of nearly $5000 on average.