DISOLUCIONES
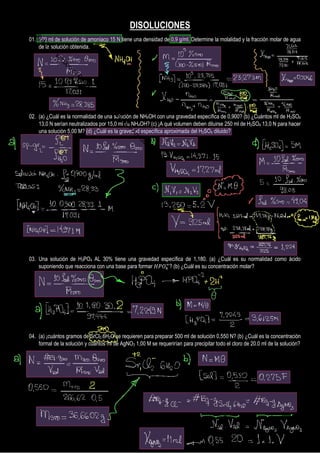
- 1. DISOLUCIONES 01. 100 ml de solución de amoniaco 15 N tiene una densidad de 0,9 g/ml. Determine la molalidad y la fracción molar de agua de la solución obtenida. 02. (a) ¿Cuál es la normalidad de una solución de NH4OH con una gravedad específica de 0,900? (b) ¿Cuántos ml de H2SO4 13,0 N serían neutralizados por 15,0 ml de NH4OH? (c) ¡A qué volumen deben diluirse 250 ml de H2SO4 13,0 N para hacer una solución 5,00 M? (d) ¿Cuál es la gravedad específica aproximada del H2SO4 diluido? 03. Una solución de H3PO4 AL 30% tiene una gravedad específica de 1,180. (a) ¿Cuál es su normalidad como ácido suponiendo que reacciona con una base para formar 𝐻𝑃𝑂4 = ? (b) ¿Cuál es su concentración molar? 04. (a) ¡cuántos gramos de SrCl2.6H2O se requieren para preparar 500 ml de solución 0,550 N? (b) ¿Cuál es la concentración formal de la solución y cuántos ml de AgNO3 1,00 M se requerirían para precipitar todo el cloro de 20.0 ml de la solución? P r o d u c e d w i t h a T r i a l V e r s i o n o f P D F A n n o t a t o r - w w w . P D F A n n o t a t o r . c o m
- 2. 05. ¿Qué fracción del peso molecular representa el peso equivalente (a) de la base Ce2O3, (b) de la sal Ca3(PO4)2 y (c) del ácido As2O5 (suponiendo reacción con una base para formar H2AsO4)? 06. ¿Cuál es la normalidad aproximada de una solución marcada con HNO3 1:4? (el HNO3 concentrado normal tiene una gravedad específica de 1.42 y contiene, aproximadamente 70% HNO3 en peso) 07. Una solución de H3PO4 contiene 0,5000 milimoles/ml, (a) ¿Cuántos ml de KOH 1,20N se requieren para convertir 5,00 ml de ácido en 𝐻2𝑃𝑂4 − ? (b) ¿A qué volumen deben diluirse 25,0 ml de ácido original para hacer la solución 1,10 N como fosfato? 08. Un paciente sufre una úlcera duodenal, producida por la concentración de 0,008 M de HCl en su jugo gástrico. Si el estómago recibe 3 L ce jugo gástrico por día, calcular la cantidad de medicamento antiácido que debe consumir si contiene 1,3 g de hidróxido de aluminio por cada 100 ml para neutralizar la acidez. P r o d u c e d w i t h a T r i a l V e r s i o n o f P D F A n n o t a t o r - w w w . P D F A n n o t a t o r . c o m
- 3. 09. Se tratan 250 g de NaCl con ácido sulfúrico concentrado (= 1,83 g/ml, 96,50% en peso de ácido sulfúrico), si el ácido se agrega con 20% en exceso, calcule el volumen de dicho ácido utilizado. Si el HCl desprendido se recoge en agua y se obtiene 500ml de solución de HCl de densidad 1,137 g/ml. Calcular la normalidad y porcentaje en peso del HCl acuoso. 10. Una muestra de sal de amoniaco que pesa 1,0090 g se calienta con KOH y el NH3 liberado se atrapa en 50 ml de una solución de ácido sulfúrico 0,5127 N. El exceso de ácido requiere 1,37 ml de álcali 0,5272 N para su titulación. Encuentre la pureza de la sal de amoniaco expresada en %Nitrógeno presente. 11. Si se agrega 50 ml de HCl 1,0870 N a 28 ml de una solución de una sustancia alcalina sólida, la última se sobreneutraliza y se requiere 10,0 ml de NaOH 0,1021 N para regresarlo al punto neutro: a) ¿Cuántos mEq contenía la solución de álcali sólida? b) ¿Cuál es la Normalidad de la solución de álcali sólido?
- 4. 12. Si una muestra de NaOH sólido está contaminado con 2,0% de Na2CO3 y 6% de H2O y se disuelve 50g de esta muestra en agua diluyéndolo hasta tener 1 L de solución, determine cuál es la normalidad de la solución como base. 13. Se debe preparar una solución de ácido oxálico para un análisis de alimento, para ello se factoriza el ácido preparado utilizando 0,0568 g de permanganato de potasio anhidro acidificando el medio con unas gotas de ácido sulfúrico concentrado, se observa virage de color y desprendimiento de dióxido de carbono. Si se factorizó una alícuota de 10ml de la solución ácida preparada, determine: a) La reacción química producida en la titulación. b) La molaridad de la solución ácida. c) Si la solución ácida se diluye de 1:5, ¿Cuál será su nueva concentración molar?
