Electronegativity Difference
•Download as PPT, PDF•
1 like•379 views
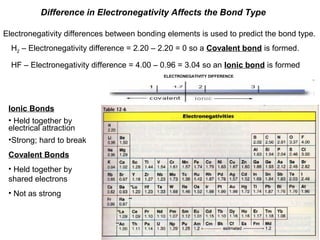
Chart depicting the electronegativity differences of atoms, including definitions and examples of ionic and covalent bonding in the context of electronegativity.
Report
Share
Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
More from Emma Wise
More from Emma Wise (20)
Cleveland's Got It All CD and the Greater Cleveland Food Bank 

Cleveland's Got It All CD and the Greater Cleveland Food Bank
How to Help the Humane Society of Central Illinois

How to Help the Humane Society of Central Illinois
Recently uploaded
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...Nguyen Thanh Tu Collection
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...christianmathematics
Mehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
Recently uploaded (20)
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...
ICT Role in 21st Century Education & its Challenges.pptx

ICT Role in 21st Century Education & its Challenges.pptx
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
Asian American Pacific Islander Month DDSD 2024.pptx

Asian American Pacific Islander Month DDSD 2024.pptx
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
Electronegativity Difference
- 1. Difference in Electronegativity Affects the Bond Type Electronegativity differences between bonding elements is used to predict the bond type. H2 – Electronegativity difference = 2.20 – 2.20 = 0 so a Covalent bond is formed. HF – Electronegativity difference = 4.00 – 0.96 = 3.04 so an Ionic bond is formed ELECTRONEGATIVITY DIFFERENCE Ionic Bonds • Held together by electrical attraction •Strong; hard to break Covalent Bonds • Held together by shared electrons • Not as strong
