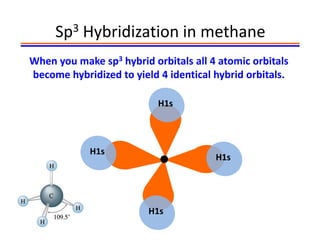
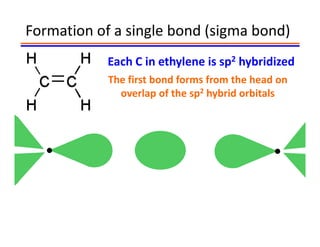
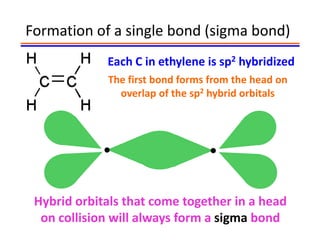
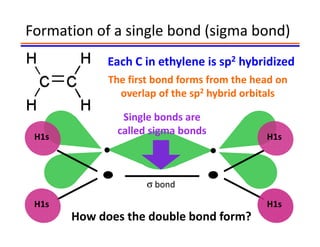
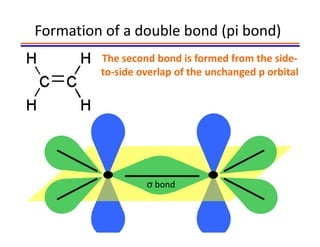
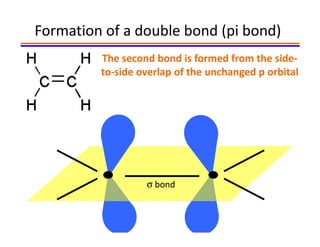
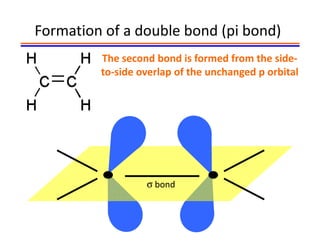
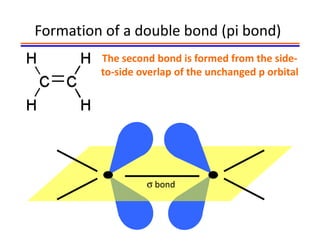
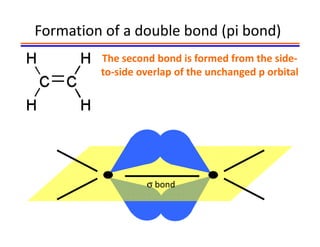
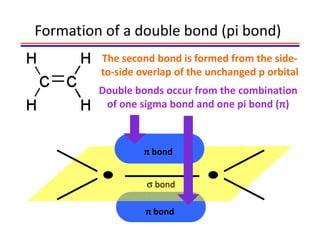
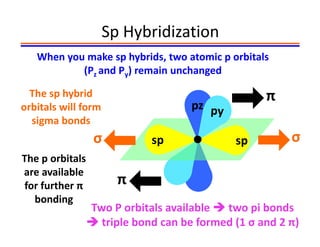
The document discusses hybridization in methane and ethylene. It explains that carbon atoms in methane are sp3 hybridized, forming 4 identical hybrid orbitals that overlap to make single sigma bonds. Carbon atoms in ethylene are sp2 hybridized, where the first bond is a sigma bond from overlap of the sp2 hybrid orbitals. The second bond is a pi bond formed from side-to-side overlap of the unhybridized p orbital. A double bond consists of one sigma bond and one pi bond. Triple bonds contain one sigma bond and two pi bonds, using the two unhybridized p orbitals.