Heat Capacity and Latent Heat.pptx
- 1. Heat Capacity and Latent Heat MOHAMED ANWER RIFKY
- 2. 1-Specific heat capacity is defined as the amount of heat required to raise the temperature of 1 kilogram of a substance by 1 kelvin (SI unit of specific heat capacity J kg-1 K-1). 2-Heat capacity is defined as the amount of heat required to raise the temperature of a given object by 1 kelvin (SI unit of heat capacity J K-1). **N.B 1 K is commensurate with 1°C. A-In a Vaporizer (component parts of different materials>>calculated heat capacity). B-Tissue specific heat capacities (mean value of 3.5 kJ kg-1 °C-1 >>a 70 kg patient ( 245 kJ °C-). C-I f temperature has fallen to 36°C>>shivering >>increase the heat production four folds to 320 W (or 240 x 60 joules per minute, i.e. 14.4 kJmin-1.) The patient ought to shiver for 17 minutes to produce the heat required. **Both core temperatures and surface skin temperatures forms >>mean body temperature. D-The specific heat of water is 4.18 kJ Kg-1 °C_1. E-1 calorie being equal to 4.18 joules. F-In dietetics the kilocalorie is often written as Calorie with a capital C. G-The specific heat of most other substances is less than that of water.The high specific heat of water is used to provide a reservoir of heat (vaporizers ). H- 2 kg of blood ( 2 litres), are transfused at 5°C and warmed up to 35°C in the patient. The heat required = 2 kg x 3.6 kJ kg-1= (2 x 3.6 x 30) kJ= 216 KJ So, the patient's mean temperature must fall by up to 1°C when 2 litre of unwarmed blood are transfused>> >Blood warmers. I-Gases have a low specific heat (1.01 kJ kg-1 °C_1) at constant pressure. 1.2 J litre-1 °C_1, ( a Thousandth) of the numerical value when expressed in terms of mass. a-An extremely small quantity of heat is required or lost when the temperature of a small volume of a gas is altered. b- Gases pass along the anaesthetict ubing, only minimal quantities of heat are transferred >> gas is closer to ambient temperature >> at the patient (humidifiers !!). c-Heat loss from warming inspired air = Flow X Specific heat capacity X Temperature rise(20 to 34 c.) = 7 litre min-1 x 1.2 J.litre-1 °C-1 x 14°C= 118 J min-1= 1.96 W (1 W = 1 J s--1). So,it is only about 2 W (Of totall of 80 W.)>> not normally an important factor. 3-LATENT HEAT: When a substance changes from a liquid to a vapour or from a solid to a liquid, heat must be supplied (at a constant temperature). latent heat of fusion is when a solid changes to a liquid. Solid out from a liquid (latent heat of crystallization). The latent heat of vaporization is of most interest in anaesthesia. Specific latent heat is defined as the heat required to convert 1 temperature (SI unit of specific latent heat Jkg-1). kilogram of a substance from one phase to another at a given A-Thus, water at 100°C may be converted to steam at 100°C by supplying the appropriate quantity of latent heat, i.e. 2.26 MJkg-1. B-At body temperature it is found that 2.42 MJ are required to turn 1 kg water into 1 kg vapour (The lower the temperature the more latent heat is needed to vaporize a substance). latent heat would continue to fall as temperature rose>>ultimately reach C-The temperature at which the latent heat of vaporization of nitrous oxide becomes zero =36.5°C( a temperature where the substance changes spontaneously from liquid to vapour without the supply of any external energy). 4-LATENT HEAT IN ANAESTHESIA: 1-Vaporization of the ethyl chloride >>pronounced cooling of the skin thus impairing conduction >> abscess or whitlow). analgesia (For the opening of a skin 2-Most modern vaporizers now have systems for controlling the concentrations of vapour based on thermostatic devices (fall in temperature of the anaesthetic in the vaporizer >>less volatile). 3-If a nitrous oxide cylinder is allowed to empty rapidly >>converted to gas, the latent heat required being taken from the remaining fluid and from the cylinder walls>>Temp.falls and water vapour from the air may condense or freeze on the outside of it.
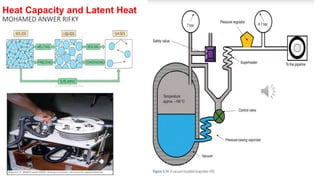
- 3. 4-As a result of cooling, the N2O vapour pressure falls rapidly inside (the pressure gauge)>>low reading which recovers towards the previous level after the cylinder is turned off (the dotted line in the fig.). 5-Carbon dioxide and cyclopropane are also stored in liquid form(in normal anaesthetic practice the rate of use is slow that cooling of the cylinder is not noticed. 6-In practice, the liquid oxygen in the storage vessel is at a temperature of around -160°C, and must be kept in a special storage vessel resembling a gigantic vacuum flask to maintain it at this low temperature (N.B O2 critical temp. is -119 C.). 1-When oxygen is taken from the top of the storage vessel, it is very cold >>superheater coil---A pressure regulator (at about 4.1 bar). 2-If oxygen flows at a fast rate>>latent heat is taken and >> vapour pressure falls>> a supplementary source of heat is needed (a pressure-raising vaporizer). A control valve senses the storage vessel pressure and controls the flow of liquid oxygen to the pressure-raising vaporizer(the lower the pressure, the higher the flow of liquid oxygen). In the vaporizer the oxygen is warmed and vaporized to the pipeline pressure. 3-If no oxygen is used ( Temperature of the storage vessel gradually rises )>> oxygen to blow off through the safety valve ,then this liquid oxygen then reduces the temperature and pressure. 5-LATENT HEAT AND HEAT LOSS FROM THE PATIENT: 1-Humidity in the upper trachea = 34 mg litre-1 2-Total water required to humidify dry air = Specific latent heat of vaporization at 37°C X Total water Specific heat to warm inspired air (calculated previously)= 2.0 W 3-Total heat loss from respiration = 11.6 W In anaesthesia, however,the inspired gases are usually dry and consequently more heat is lost by this route than under normal conditions(15% of the total basal heat loss of about 80 W.Instead of the 10% given in Chapter 9). 4-The older closed anaesthetic system with the Waters canister >> efficient conservation of a patient's heat and moisture other sources of heat loss are blocked by placing the person in a wax bath,and as a in the treatment of cancer.
