5 FUELS.ppt
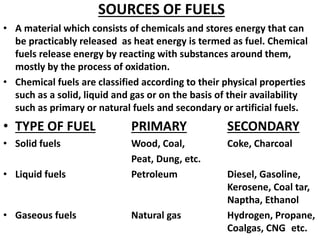
- 1. SOURCES OF FUELS • A material which consists of chemicals and stores energy that can be practicably released as heat energy is termed as fuel. Chemical fuels release energy by reacting with substances around them, mostly by the process of oxidation. • Chemical fuels are classified according to their physical properties such as a solid, liquid and gas or on the basis of their availability such as primary or natural fuels and secondary or artificial fuels. • TYPE OF FUEL PRIMARY SECONDARY • Solid fuels Wood, Coal, Coke, Charcoal Peat, Dung, etc. • Liquid fuels Petroleum Diesel, Gasoline, Kerosene, Coal tar, Naptha, Ethanol • Gaseous fuels Natural gas Hydrogen, Propane, Coalgas, CNG etc.
- 2. COAL • Coal, a mineral of fossilized carbon, is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers called coal beds. The harder forms, such as anthracite coal ( C- 92% -98%), can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure. • Coal is composed primarily of carbon along with variable quantities of other elements, chiefly Hydrogen, Sulphur, Oxygen, and Nitrogen. • Coal has been a useful resource and it is primarily used for the production of electricity or heat, and is also used for industrial purposes, such as refining metals.
- 3. TYPES OF COAL • PEAT • Peat has industrial importance as a fuel in some regions like Ireland and Finland. • In its dehydrated form, peat is a highly effective absorbent for fuel and oil spills on land and water. • It is also used as a conditioner for soil to make it able to retain more water. • Used for cooking & domestic heating. Gardeners use peat moss mainly as a soil amendment or ingredient in potting soil. It has an acid pH, so it's ideal for acid loving plants, such as blueberries and camellias.
- 4. lignite • Lignite or brown coal, is the lowest rank of coal and used almost exclusively as fuel for electric power generation. • Jet, a compact form of lignite, is sometimes polished and has been used as an ornamental stone. • It is a softer coal with a high moisture content and contains carbon ,sulphur & mercury
- 5. • The carbon content of lignite ranges from 65- 70% and represents the youngest rank of fuel—with approximate ages of around 60 million years. Approximately 7 percent of the coal mined in the U.S. is lignite. • It's found primarily in North Dakota , Texas • Mississippi .
- 6. • Peat is the first step in coal formation. Peat is composed of over 60% organic matter; • Lignite is a soft brown coal that still contains a high amount of water. • Lignite has a higher heat content than peat but is still not the most desired form of coal.
- 7. TYPES OF COAL (CONTD. – 1) • SUB-BITUMINOUS COAL • It’s properties range from those of lignite to those of bituminous coal and is primarily used as fuel for steam- electric power generation and is an important source of light aromatic hydrocarbons for the chemical synthesis industry. • BITUMINOUS COAL • It is a dense sedimentary rock, usually black or dark brown, often with well-defined bands of bright and dull material. It is primarily used as fuel in steam-electric power generation, with substantial quantities used for heat and power applications in manufacturing and to make coke.
- 8. TYPES OF COAL (CONTD. – 2) • ANTHRACITE • It is the best grade of coal, which is a harder, glossy black coal used primarily for residential and commercial space heating. It may be divided further into metamorphically altered bituminous coal and "petrified oil“. • GRAPHITE • It is technically the highest grade but it is difficult to ignite thus it is not commonly used as fuel. It is mostly used in pencils and, when powdered, as a lubricant.
- 9. COMPARISON OF COALS GRADE % VOLA. C% H2% O2% S% Heat kJ/kg Lignite 45–65 60–75 6.0–5.8 34-17 0.5-3 <28,470 Flame Coal 40-45 75-82 6.0-5.8 >9.8 ~1 <32,870 Gas Flame 35-40 82-85 5.8-5.6 9.8-7.3 ~1 <33,910 Coal Gas Coal 28-35 85-87.5 5.6-5.0 7.3-4.5 ~1 <34,960 Fat Coal 19-28 87.5-89.5 5.0-4.5 4.5-3.2 ~1 <35,380 Forge Coal 14-19 89.5-90.5 4.5-4.0 3.2-2.8 ~1 <35,380 Non-Baking 10-14 90.5-91.5 4.0-3.75 2.8-3.5 ~1 35,380 Coal Anthracite 7-12 >91.5 <3.75 <2.5 ~1 <35,300
- 10. PETROLEUM • Petroleum is a naturally occurring flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. • The word Petroleum is referred for both the naturally occurring unprocessed crude oils and petroleum products that are made up of refined crude oil. • Petroleum is recovered mostly through oil drilling. It is refined and separated easily by boiling into a large number of consumer products such as petrol or gasoline, kerosene to asphalt and chemical reagents used to make plastics and pharmaceuticals.
- 11. The World's Largest Oil Reserves By Country • Venezuela - 300,878 million barrels. • Saudi Arabia - 266,455 million barrels. ... • Canada - 169,709 million barrels. ... • Iran - 158,400 million barrels. ... • Iraq - 142,503 million barrels. ... • Kuwait - 101,500 million barrels. ... • United Arab Emirates - 97,800 million barrels. Russia - 80,000 million barrels. ... •
- 12. PETROLEUM (CONTD. – 1) • In strict sense, petroleum includes only crude oil, but it includes all liquid, gaseous, and solid hydrocarbons. The lighter hydrocarbons such as methane, ethane, propane and butane occur as gases, while pentane and heavier ones are in the form of liquids or solids. • An oil well produces predominantly crude oil, with some natural gas dissolved in it. Heavier hydrocarbons such as pentane, hexane, and heptane etc. are in gaseous form under the earth’s crust but condense at normal pressure and temperature on extraction. Condensate resembles petrol in appearance & similar in composition to other volatile light crude oils.
- 13. PETROLEUM (CONTD. – 2) • The proportion of light hydrocarbons in the petroleum mixture varies greatly among different oil fields, ranging from as much as 97 percent by weight in the lighter oils to as little as 50 percent in the heavier oils and bitumens. • The hydrocarbons in crude oil are mostly alkanes, cyclo-alkanes and various aromatic hydrocarbons while the other organic compounds contain nitrogen, oxygen and sulphur, and trace amounts of metals such as iron, nickel, copper and vanadium.
- 14. COMPOSITION OF PETROLEUM ELEMENT RANGE Carbon 83 to 87% Hydrogen 10 to 14% Nitrogen 0.1 to 2% Oxygen 0.05 to 1.5% Sulphur 0.05 to 6.0% Metals < 0.1% HYDROCARBON RANGE Paraffins 15 to 60% Naphthenes 30 to 60% Aromatics 3 to 30% Asphaltics 6%
- 15. USES OF PETROLEUM • Petroleum is used mostly for producing fuel oil and petrol. 84 % of the hydrocarbons present in petroleum is converted into energy-rich fuels including petrol, diesel, jet, heating, and other fuel oils & liquefied petroleum gas. • Petroleum is also the raw material for many chemical products, including pharmaceuticals, solvents, fertilizers, pesticides, and plastics. Remaining 16 % hydrocarbon is converted into these materials. • The lighter grades of crude oil provide best products, but the world's reserves of light and medium oil have been depleted. Oil refineries now use complex and expensive methods to use heavier crude oils which have too much carbon and not enough hydrogen using fluid catalytic cracking to convert the longer, more complex molecules in the oil to the shorter, simpler ones in the fuels.
- 16. USES OF PETROLEUM (CONTD. – 1) • Certain types of hydrocarbons may be mixed with other non-hydrocarbons, to create other products. • Alkenes can be used for plastics or other compounds. • Lubricants such as light machine oils, motor oils, and greases by adding viscosity stabilizers. • Wax is used in the packaging of frozen foods. • Sulphur or Sulphuric acid are useful industrial materials obtained as a byproduct of sulphur removal from fuels. • Bulk tar. • Asphalt • Petroleum coke, used as solid fuel. • Aromatic petrochemicals used in other chemical production
- 17. • Alkenes are Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. • Those with at least one carbon-to-carbon double bond are called alkenes and those with at least one carbon-to-carbon triple bond are called alkynes.
- 18. Cracking of crude oil • In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon- carbon bonds in the precursors.
- 19. • Cracking allows large hydrocarbon molecules to be broken down into smaller, more useful hydrocarbon molecules. Fractions containing large hydrocarbon molecules are heated to vaporise them.
- 20. Refining process • Cracking processes break down heavier hydrocarbon molecules (high boiling point oils) into lighter products such as petrol and diesel. • These processes include • Catalytic cracking, • Thermal cracking and • Hydro-cracking.
- 21. • Catalytic cracking is used to convert heavy hydrocarbon fractions obtained by vacuum distillation into a mixture of more useful products such as petrol and light fuel oil. • In this process, the feedstock undergoes a chemical breakdown, under controlled heat (450 – 500oC) and pressure, in the presence of a catalyst –
- 22. • CATALYST is a substance which promotes the reaction without itself being chemically changed. • Small pellets of silica – alumina or silica – magnesia have proved to be the most effective catalysts.
- 23. The cracking reaction yields • Petrol, LPG, Unsaturated olefin compounds, Cracked gas oils, Light gases and a Solid coke residue. A liquid residue called cycle oil, and a solid coke residue. • Cycle oil is recycled to cause further breakdown and the coke, which forms a layer on the catalyst, is removed by burning. • Other products passed through a fractionator to be separated and separately proces
- 24. • Fluid catalytic cracking uses a catalyst in the form of a very fine powder which flows like a liquid when agitated by steam, air or vapour. • Feedstock entering the process immediately meets a stream of very hot catalyst and vaporises. • The resulting vapours keep the catalyst fluidised as it passes into the reactor, where the cracking takes place and where it is fluidised by the hydrocarbon vapour.
- 25. • The catalyst next passes to a steam stripping section where most of the volatile hydrocarbons are removed. • It then passes to a regenerator vessel where it is fluidised by a mixture of air and the products of combustion which are produced as the coke on the catalyst is burnt off. • The catalyst then flows back to the reactor.
- 26. • The catalyst thus undergoes a continuous circulation between the reactor, stripper and regenerator sections. • The catalyst is usually a mixture of aluminium oxide and silica. • Most recently, the introduction of synthetic zeolite catalysts has allowed much shorter reaction times and improved yields and octane numbers of the cracked gasolines.
- 27. THERMAL CRACKING • Thermal cracking uses heat to break down the residue from vacuum distillation. • The lighter elements produced from this process can be made into distillate fuels and petrol. • Cracked gases are converted to petrol blending components by alkylation or polymerisation.
- 28. • Naphtha is upgraded to high quality petrol by reforming. • Gas oil can be used as diesel fuel or can be converted to petrol by hydrocracking. • The heavy residue is converted into residual oil or coke which is used in the manufacture of electrodes, graphite and carbides.
- 29. • Hydrocracking can increase the yield of petrol components, as well as being used to produce light distillates. It produces no residues, only light oils. Hydrocracking is catalytic cracking in the presence of hydrogen. • The extra hydrogen saturates, or hydrogenates, the chemical bonds of the cracked hydrocarbons and creates isomers with the desired characteristics
- 30. • . Hydrocracking is also a treating process, because the hydrogen combines with contaminants such as sulphur and nitrogen, allowing them to be removed. Gas oil feed is mixed with hydrogen, heated, and sent to a reactor vessel with a fixed bed catalyst, where cracking and hydrogenation take place. • Products sent to fractionator for separation.
- 31. • The hydrogen is recycled. Residue from this reaction is mixed again with hydrogen, reheated, and sent to a second reactor for further cracking under higher temperatures and pressures.
- 32. • In addition to cracked naphtha for making petrol, hydrocracking yields light gases useful for refinery fuel, or alkylation as well as components for high quality fuel oils, lube oils and petrochemical feedstocks. • Following the cracking processes it is necessary to build or rearrange some of the lighter hydrocarbon molecules
- 33. • into high quality petrol or jet fuel blending components or into petrochemicals. • The former can be achieved by several chemical process such as alkylation and isomerisation.
- 35. FRACTINATOR
- 36. CRACKING OF MINERAL CRUDE OIL • Fluid catalytic cracking is a commonly used process in a modern oil refinery. The process was first used around 1942 and employs a powdered catalyst mainly consisting of aluminium oxide and silica into small, porous pieces. • Pre-heated feed is sprayed into the base of the riser via feed nozzles where it contacts extremely hot fluidized catalyst at 666 to 760 °C. • The hot catalyst vaporizes the feed & catalyzes the cracking reactions that break down the high-molecular weight oil into lighter components including LPG, gasoline, and diesel.
- 37. CRACKING OF MINERAL CRUDE OIL (CONTD. – 1) • The catalyst-hydrocarbon mixture flows upward through the riser for a few seconds, and then the mixture is separated via cyclones. • The catalyst-free hydrocarbons are routed to a main fractionator for separation into fuel gas, LPG, gasoline, naphtha, light cycle oils used in diesel and jet fuel, and heavy fuel oil. • During the trip up the riser, the cracking catalyst is used up reactions which deposit coke on the catalyst and greatly reduce activity and selectivity.
- 38. CRACKING OF MINERAL CRUDE OIL (CONTD. – 2) • Used up catalyst is separated from the cracked hydrocarbon vapors and sent to a stripper where it contacts steam to remove hydrocarbons remaining in the catalyst pores. • Used up catalyst then flows into a fluidized-bed regenerator where air is used to burn off the coke to restore catalyst activity and also provide the necessary heat for the next reaction cycle, cracking being an endothermic reaction. • The regenerated catalyst then flows to the base of the riser, repeating the cycle.
- 39. CHARACTERISTICS OF MARINE FUELS MARINE DISTILLATE FUELS Parameter Unit Limit DMX DMA DMB DMC Density at 15 °C kg/m³ Max - 890.0 900.0 920.0 Viscosity at 40 ° C mm²/s Max 5.5 6.0 11.0 14.0 Viscosity at 40 °C mm²/s Min 1.4 1.5 - - Micro Carbon Residue % m/m Max 0.30 0.30 - - at 10% Residue Micro Carbon Residue % m/m Max - - 0.30 2.50 Water % V/V Max - - 0.3 0.3 Sulphur c % m/m Max 1.0 1.5 2.0 2.0 Total Sediment Existent % m/m Max - - 0.10 0.10 Ash % m/m Max 0.01 0.01 0.01 0.05 Vanadium mg/kg Max - - - 100 Aluminium + Silicon mg/kg Max - - - 25 Flash point °C Min 43 60 60 60
- 40. CHARACTERISTICS OF MARINE FUELS (CONTD. - 1) MARINE DISTILLATE FUELS (CONTD. – 1) Parameter Unit Limit DMX DMA DMB DMC Pour point, Summer °C Max - 0 6 6 Pour point, Winter °C Max - -6 0 0 Cloud point °C Max -16 - - - Calculated Cetane Index Min 45 40 35 - Appearance Clear & Bright - - Zinc d mg/kg Max - - - 15 Phosphorus d mg/kg Max - - - 15 Calcium d mg/kg Max - - - 30 c (A sulphur limit of 1.5% m/m will apply in SOx Emission Control Areas designated by the International Maritime Organization. There may be local variations.) d (The Fuel shall be free of ULO. A Fuel is considered to be free of ULO if one or more of the elements are below the limits. All three elements shall exceed the limits before deemed to contain ULO.)
- 41. CHARACTERISTICS OF MARINE FUELS (CONTD. - 2) MARINE RESIDUAL FUELS Parameter Unit Limit RME RMF RMG RMH 180 180 380 380 Density at 15 °C kg/m³ Max 991.0 991.0 991.0 991.0 Viscosity at 50°C mm²/s Max 180.0 180.0 380.0 380.0 Water % V/V Max 0.5 0.5 0.5 0.5 Micro Carbon Residue % m/m Max 15 20 18 22 Sulphur c % m/m Max 4.50 4.50 4.50 4.50 Ash % m/m Max 0.10 0.15 0.15 0.15 Vanadium mg/kg Max 200 500 300 600 Flash point °C Min 60 60 60 60 Pour point, Summer °C Max 30 30 30 30 Pour point, Winter °C Max 30 30 30 30 Aluminium + Silicon mg/kg Max 80 80 80 80 Total Sediments % m/m Max 0.10 0.10 0.10 0.10
- 42. CHARACTERISTICS OF MARINE FUELS (CONTD. - 3) MARINE RESIDUAL FUELS Parameter Unit Limit RME RMF RMG RMH 180 180 380 380 • Zinc d mg/kg Max 15 15 15 15 • Phosphorus d mg/kg Max 15 15 15 15 • Calcium d mg/kg Max 30 30 30 30 • c A sulphur limit of 1.5% m/m will apply in Sox Emission Control Areas designated by the International Maritime Organization. There may be local variations. • d The Fuel shall be free of ULO. A Fuel is considered to be free of ULO if one or more of the elements are below the limits. All three elements shall exceed the limits before deemed to contain ULO.
- 43. ORIMUSLION • Orimulsion is a registered trade name for a bitumen based fuel developed for industrial use by Venezuela. • Like coal & oil, bitumen occurs naturally & obtained from the world's largest deposit in the Orinoco in Venezuela. • Raw bitumen has an extremely high viscosity and specific gravity and is unsuitable for direct use in conventional power stations. • Orimulsion is made by mixing the bitumen with about 30% fresh water and a small amount of surfactant which behaves similarly to fuel oil.
- 44. ORIMUSLION (CONTD. -1) • ADVANTAGES OF ORIMUSLION • As a fuel for electricity generation, Orimulsion is very competitive with coal. • It is relatively easy and safe to produce, transport, handle and store. • It is easy to ignite & has good combustion characteristics. • It can be used in power stations designed to run on coal or heavy fuel oil, with suitable modification.
- 45. ORIMUSLION (CONTD. -2) • DISADVANTAGES OF ORIMUSLION • If there is a spill in water during it’s transportation, the mixture de-emulsifies and the bitumen comes out of suspension. • It is a non-Newtonian fluid, thus if it is allowed to cool below 30 °C, it will set and pumping becomes impossible, there is no way of restarting flow through pipeline. • During a strike, supplies of orimulsion were disrupted, as a result, except China and Cuba, it’s use was discontinued. • Due to rising crude oil prices, Orinoco bitumen (extra heavy oil) is being diluted with a lighter crude oil which makes this blend more profitable as a crude oil in the world market than by selling it as Orimulsion.
- 46. NATURAL GAS • Natural gas is a naturally occurring hydrocarbon gas mixture consisting primarily of methane, but commonly including varying amounts of other hydrocarbons, carbon dioxide, nitrogen and hydrogen sulphide. • Natural gas is an energy source often used for heating, cooking, and electricity generation. • Also used as fuel for vehicles and as a chemical feedstock in the manufacture of plastics and other commercially important organic chemicals. • Natural gas is found in deep underground natural rock formations or associated with other hydrocarbon reservoirs in coal beds.
- 47. • Most natural gas is created over time by two Mechanisms namely • Biogenic and • Thermogenic. • Biogenic gas is created by methanogenic • organisms in marshes, bogs, landfills, and shallow sediments.
- 48. • THERMOGENIC GAS:- Deeper in the earth, at greater temperature and pressure, thermogenic gas is created from buried organic material. • Before natural gas can be used as a fuel, it must undergo processing to remove • impurities, including water, to meet the specifications of marketable natural gas.
- 49. • Byproducts of processing include ethane, propane, butanes, pentanes, and higher molecular weight hydrocarbons, hydrogen sulphide, carbon dioxide, water vapour, and sometimes helium and nitrogen. • Natural gas is often called as gas, especially when compared to other energy sources such as oil or coal.
- 50. NATURAL GAS (CONTD. – 2) • In the 19th century natural gas was usually obtained as a by- product of producing oil and was unwanted and virtually valueless since it had to be piped to the end user thus gas was burned off at oil fields. • Nowadays pipelines are constructed when it is economically feasible to transport gas from a wellsite to an end consumer in the regions where natural gas has high demand. • Natural gas is also exported as a liquid and transported to users through conventional pipelines and tankers. • Apart from oil fields, natural gas is also associated with Coal beds or Natural gas fields.
- 51. • It sometimes contains a significant amount of ethane, propane, butane, and pentane, after removal of heavier hydrocarbons for commercial use prior to the methane being sold as a consumer fuel or chemical plant feedstock. • Non-hydrocarbons such as carbon dioxide, nitrogen, and hydrogen sulfide are removed before the natural gas is transported.
- 52. NATURAL GAS (CONTD. – 3) • TOWN GAS or coal gas refers to a gaseous mixture, used as a fuel, that is released when bituminous coal is burned. • Town gas is a flammable gaseous fuel made by the destructive distillation of coal and contains a variety of calorific gases. • including hydrogen, carbon monoxide, methane and other volatile hydrocarbons together with small quantities of non- calorific gases such as carbon dioxide and nitrogen. • It is used in a similar way to natural gas. • Though not economically competitive with other sources of fuel, but there are some specific cases where it is the best option and it may be so into the future.
- 53. • Town gas produced in gas houses is a by- product of coke ovens in which heated bituminous coal in air-tight chambers. • The coal tar or asphalt collected at the bottom of the gashouse ovens is used for roofing and other water-proofing purposes & when mixed with sand and gravel was used for paving streets.
- 54. NATURAL GAS (CONTD. – 4) • BIOGAS • When methane-rich gases are produced by the anaerobic decay of non-fossil organic matter commonly called biomass, product is referred to as biogas. Sources of biogas include swamps, marshes, and landfills, sewage sludge and manure by way of anaerobic digestion. • Methane released directly into the atmosphere acts as a pollutant as it gets oxidized, producing carbon dioxide and water. Methane in the atmosphere has a half life of seven years, 50% methane broken down to carbon dioxide and water after seven years. • It is economical to consume the gas on site or within a short distance of the landfill using a dedicated pipeline. Water vapor is often removed, even if the gas is combusted on site. Other non- methane components are removed to prevent fouling of the equipment or the environmental. Gas produced in sewage plants is usually consumed to generate electricity.
- 55. FLOW CHART OF NATURAL GAS GENERATION
- 56. NATURAL GAS (CONTD. – 5) • Natural gas is a major source of electricity generation through the use of cogeneration (Gas turbines and steam turbines). Natural gas is also well suited for a combined use in association with renewable energy sources such as wind or solar and for supplementing peak-load in power stations functioning in tandem with hydroelectric plants. • High efficiencies can be achieved through combining gas turbines with a steam turbine in combined cycle mode. Natural gas burns more cleanly than other hydrocarbon fuels and produces 30% less carbon dioxide per unit of energy released. Natural gas powered Combined Heat and Power plant (Cogeneration plant) is considered the cleanest and energy efficient available source and a rapid way to cut carbon emissions.
- 57. PROPERTIES OF MARINE FUELS • KINEMATIC VISCOSITY • Kinematic viscosity is a measure for the fluidity of the product at a certain temperature. • The viscosity of a fuel decreases with increasing temperature. • The viscosity at the moment the fuel leaves the injectors has to be within the limits prescribed by the engine manufacturers to obtain an optimal spray pattern. • Viscosity outside manufactures specifications at the injectors will lead to Poor combustion Deposit formation and Energy loss.
- 58. • The viscosity of the fuel has to be such that the required injection viscosity can be reached by the ship’s preheating system.
- 59. PROPERTIES OF MARINE FUELS (CONTD. – 1) • DENSITY • The official unit is kg/m3 at 15°C, while kg/l at 15°C is the most commonly used unit. • Density is used in the calculation of the quantity of fuel delivered. • From a technical point of view, the density gives an indication of the ignition quality of the fuel within a certain product class, this is particularly the case for the low viscosity IFOs. • The product density is important for the onboard purification of the fuel. • The higher the density the more critical it becomes for purification.
- 60. PROPERTIES OF MARINE FUELS (CONTD. - 2) • CETANE NUMBER AND CETANE INDEX • Only applicable for gasoil and distillate fuels. • It is a measure of ignition quality of the fuel in a diesel engine. • The higher the rpm of the engine, the higher the required Cetane number. The Cetane number is determined on an engine. • The Cetane index is an approximate value of the Cetane number based on the density and the distillation of the fuel. The Cetane index is not applicable when Cetane improving additives are
- 61. PROPERTIES OF MARINE FUELS (CONTD. - 3) • CARBON RESIDUE • A carbon residue determination is a typical laboratory test performed under specified reduced air supply and does not represent combustion conditions in an engine. • It gives an indication of the amount of hydrocarbons in the fuel which have difficult combustion characteristics, but there is no conclusive correlation between carbon residue figures and actual field experience. The micro carbon residue method is specified by ISO 8217. • ASH • The ash content is a measure of the metals present in the fuel, either as inherent to the fuel, or as contamination.
- 62. • Conradson carbon residue, commonly known as "Concarbon" or "CCR" is a laboratory test used to provide an indication of the coke- forming tendencies of an oil. Quantitatively, the test measures the amount of carbonaceous residue remaining after the oil's evaporation and pyrolysis. For diesel fuel, Concarbon correlates approximately with combustion chamber deposits,
- 63. PROPERTIES OF MARINE FUELS (CONTD. - 4) • FLASH POINT • Flash point is the temperature at which the vapours of a fuel ignite (under specified test conditions), when a test flame is applied. The flash point for all fuels to be used in bulk onboard vessels is set at PM, CC, 60°C minimum (SOLAS agreement). DMX, a special low cloud point gasoil, may only be stored onboard in drums because of its <60°C flash point. • SULPHUR • The sulphur content of a marine fuel depends on the crude oil origin and the refining process. When a fuel burns, sulphur is converted into sulphur oxides. These oxides reach the lubricating oil via the blow-by gas . These oxides are corrosive to engine cylinder liners and must be neutralized by the cylinder lubricant.
- 64. PROPERTIES OF MARINE FUELS (CONTD. – 5) • Marine engine lubricants are developed to cope with this acidity (high BN). If the correct lubricant is used, the sulphur content of a marine fuel is technically not important (although it has environmental implications in sensitive areas such as the Baltic Sea). • WATER CONTENT • Water in fuel is a contamination and does not yield any energy. The percentage of water in the fuel can be translated into a corresponding energy loss for the customer. Water is removed onboard the vessel by centrifugal purification. If after purification the water content remains too high, water vapour lock can occur and pumps can cut out.
- 65. PROPERTIES OF MARINE FUELS (CONTD. – 6) • If water-contaminated fuel reaches the injectors, combustion can be erratic. Water in fuel which remains standing in lines for a longer period can cause corrosion. • POUR POINT • Pour point is the lowest temperature at which a fuel will continue to flow when it is cooled under specified standard conditions. Contrary to straight run type heavy fuels (pour point typically in the +20°C range), bunker fuels from a complex refinery generally have pour points below 0°C (range –10 to –20°C).
- 66. PROPERTIES OF MARINE FUELS (CONTD. - 7) • This is reflected in the fact that bunker fuel tanks are generally not completely heated any more, but only before the fuel transfer pump. This can then lead to problems, if a vessel receives high pour straight run bunker fuel. • For distillate marine diesel, cold temperature behaviour is controlled in ISO 8217 by maximum pour point. With marine diesels, a high content of heavier n-paraffins vigilance is required if strong temperature changes are expected resulting in settling of wax even when pour point specs have been met.
- 67. Most of the paraffin compounds in naturally occurring crude oils are normal paraffins, while isoparaffins are frequently produced in refinery processes. The normal paraffins are uniquely poor as motor fuels, while isoparaffins have good engine-combustion characteristics.
- 68. PROPERTIES OF MARINE FUELS (CONTD. - 8) • ELEMENTS • Vanadium and nickel are elements found in certain heavy fuel oil molecules (asphaltenes). Upon combustion Vanadium oxides are formed and some of them have critical melting temperatures. The most critical are the double oxides / sulphates with sodium. Some countries have implemented maximum Ni concentrations for inland use of heavy fuel due to emission regulations. • TOTAL SEDIMENT POTENTIAL • Inorganic material naturally occurring in crude oil is removed in the refineries prior to the atmospheric distillation. Some minor contamination (e.g. Iron oxides) of a finished heavy fuel can not be excluded.
- 69. • Asphaltenes consist primarily of carbon, hydrogen, nitrogen, oxygen, and sulfur, as well as trace amounts of vanadium and nickel. The C:H ratio is approximately 1:1.2, depending on the asphaltene source.
- 70. PROPERTIES OF MARINE FUELS (CONTD. - 9) • The biggest risk for sediment formation in heavy fuel is due to potential coagulation of organic material inherent to the fuel itself (viz.broken asphaltenes), if insufficiently stable, can form sediment (coagulation is influenced by time and temperature). A decrease in aromaticity of the fuel matrix by blending with paraffinic cutterstocks can also deteriorate the stability of the asphaltenes. • In cases of heavy fuel instability, it is only a relative small fraction of the asphaltenes which forms sediment, but this organic sediment includes in its mass some of the fuel itself and water. The amount of generated sludge can become quite high. The total potential sediment gives the total amount of sediment that can be formed under normal storage conditions, excluding external influences.
- 71. ASPHALTENE CONTAMINATION Some of the symptoms are as followed: • Filter Plugging • Reduced fuel economy • Loss of power • More frequent injector replacements • More frequent filter changes • More money out of pocket!
- 72. • Cutter stocks are generally lighter petroleum liquids than the heavy fuel oil produced by Russian refineries, which can be used to reduce viscosity to produce on- specEuropean fuel oil. The bulk of Europe's cutter stock supply comes from US refineries. • Kerosine is cutterstock oil
- 73. • Straight run fuel oil is the residue that comes out from the distillation column, without further processing in vaccum distillation unit or residue catalytic cracker. •
- 74. PROPERTIES OF MARINE FUELS (CONTD. - 10) • If the total potential sediment of the heavy fuel oil markedly exceeds the specification value (0.10 % (m/m) max for all grades of IFOs and HFOs, problems with the fuel cleaning system can occur, fuel filters can get plugged and combustion become erratic. • CATALYTIC FINES • As described previously, HCO is used worldwide in complex refining as a blending component for heavy fuel. Mechanically damaged catalyst particles (aluminum silicate) cannot be removed completely in a cost-effective way, and are found in blended heavy fuel.
- 75. PROPERTIES OF MARINE FUELS (CONTD. - 11) • Fuel precleaning onboard ships has a removal efficiency of approximately 80% for catalyst fines. In order to avoid abrasive wear of fuel pumps and injectors, a maximum limit of 80 mg/kg for Al+Si has been defined in ISO 8217. • CALCULATED CARBON AROMATICITY INDEX (CCAI) • CCAI is an indicator of the ignition delay of an IFO.CCAI is calculated from the density and the viscosity of the fuel oil. Although it is not an official specification, it has found its way in many users’ bunker fuel specification requirements. Some manufacturers specify CCAI limits for their engines, depending on engine type and application.
- 76. UNDESIRED ELEMENTS IN MARINE FUELS • One of the greatest sources of trouble of fuel oils is the water and sediments often present in the oil. Following sediments are commonly seen in fuel oils. • Vanadium and Nickel are present in crude oils which are carried into marine fuels. • Aluminium and Silicon are used as catalysts in Refineries and available in fuels as catalytic fines and are highly abrasive. • Sodium is freely available in marine fuels and is highly corrosive in combination. • Shipboard fuel oil purification is aimed to remove most of these unwanted substances from marine fuels.
- 77. • 6. Abrasion: The heavy fuel oil contains deposits such as vanadium, sulphur, nickel, sodium, silicon etc. which are difficult to remove and have an abrasive effect on the liner and piston surfaces. • 7. Corrosion: Elements such as vanadium and sulphur, which are present in the heavy fuel oil, leads to high temperature and low- temperature corrosion respectively. •
- 78. • Sulphur is also present in the heavy grade fuel. When sulphur combines with oxygen to form sulphur dioxide or sulphur trioxide, it further reacts with moisture (which can be due to low load operation) to form vapours of sulphuric acid. When the metal temperature is below the dew point of acid, the vapours condense on the surface and cause low-temperature corrosion.
- 79. • Vanadium when comes in contact with sodium and sulphur during the combustion, forms a eutectic compound with a low melting point of 530°C. • This molten compound is highly corrosive and attacks the oxide layers on the steel liner and piston (which is used to protect the steel surface), leading to corrosion.
- 80. TREATMENT OF MARINE FUELS ON BOARD • In the late 70’s, the increasing market demand for distillate type fuels (gasoline, diesel) and the resulting changes in refinery processes to cope with this demand have resulted in a deterioration of the heavy fuel quality. • Efficient cleaning of heavy fuel oil is mandatory to achieve reliable and economical operation of diesel engines burning heavy fuel. • Water is a common contaminant in fuel oil. Apart from water content in the fuel oil, during transportation there can be a further contamination in the storage tank due to water condensation as a result of temperature changes. • Catalyst fines from aluminium silicate used in the catalytic cracking process may end up in the heavy fuel and have to be removed to avoid abrasive wear of various engine parts.
- 81. TREATMENT OF MARINE FUELS ON BOARD (CONTD. – 1) • Treatment on board ship involves removal of Impurities from the fuel oil by Separation and Filtration. • Separation is carried out both by gravitation in settling tank and centrifugal in purifier/clarifier. • Filtration is done in two or three stages and fineness of filter depends on the size of nozzle through which the oil has to pass which in turn depends on degree of atomization and penetration required. • In diesel engines, the combustion chamber is at high pressure and time available for ignition is very short’
- 82. • Thus fuel should reach the combustion chamber in a finely atomized form, achieved by pumping fuel at high pressure and passing it through very fine holes. • This will require a notch wire filter separating impurities to very small size.
- 83. TREATMENT OF MARINE FUELS ON BOARD (CONTD. – 2) • On the other hand, pressure in the boiler furnace is near atmospheric and time available for combustion is sufficient thus oil under moderate pressure can penetrate through the furnace and very fine atomization is not required. • As the burner tip for the boiler has large holes, course filtration is sufficient to prevent it’s blockage. Contrary to diesel engine where impurities leave deposits on piston crown and increase the abrasive wear on running surface, boiler combustion is not affected by the presence of impurities thus centrifuging is considered unnecessary. • Boiler fuel just needs to be settled for removal of water & large impurities whereas treatment of diesel engine requires, sedimentation, separation & filtration.
- 84. TREATMENT OF MARINE FUELS ON BOARD (CONTD. – 3) • Fuel from the storage tank is pumped to the settling tank and contaminants (WATER & SOILDS) sink to the bottom of the tank under influence of the gravity force (g). • The rate of separation by gravity, Vg is defined by Stoke’s law: Vg = [d2 (ρ2 - ρ1) / 18 η] g, where d = particle diameter ρ2 = particle density ρ1 = density of the fuel oil η = viscosity of the fuel oil g = gravitational acceleration. • Complete separation in a reasonable period of time can only be achieved by mechanically generated centrifugal force.
- 85. TREATMENT OF MARINE FUELS ON BOARD (CONTD. – 4) • Fuel from the settling tank is fed to a centrifugal system or purifier and water and solids are separated out of the fuel. The rate of separation in a centrifugal field (V) is defined as: • V= Vg x Z where Z = rω2/g r = distance of the particle from the axis of rotation ω = angular velocity • The factor Z specifies how much greater the sedimentation rate is in the centrifugal field compared to the gravitational field.
- 86. CHANGE IN DENSITY WITH INCREASE IN TEMPERATURE
- 87. USE OF CENTRIFUGES • In a purifier type separator, cleaned oil and separated water are continuously discharged during operation. An interface is formed in the bowl between the water and the oil. This interface position is affected by several factors, such as density and viscosity of the fuel oil, temperature and flow rate. • Position of the interface becomes progressively more sensitive with increasing fuel density. The generally accepted maximum density limit for the conventional purifier is 991 kg/m3 (at 15°C). • In order to achieve optimum separation results with purifiers, the interface between oil and water in the bowl must be outside the disc stack but at the inside of the outer edge of the top disc.
- 88. USE OF CENTRIFUGES (CONTD. – 1) PURIFIER BOWL ARRANGEMENT
- 89. USE OF CENTRIFUGES (CONTD. – 2) • The position of the interface is affected mainly by the DENSITY and the VISOSITY of the fuel oil and is adjusted by means of gravity discs. • The correct gravity disc is defined as the largest disc that does not cause a broken water seal. With the correct interface position, the oil feed can enter the narrow channels. • For fuel oils with a viscosity above 180 mm2/s at 50°C, it is recommended that the highest possible temperature (98°C) be maintained. The fuel oil has to remain in the centrifuge bowl for as long as possible by adjusting the flow rates through the centrifuge so that it corresponds to the amount of fuel required by the engine. • If the interface is in an incorrect position the oil to be cleaned will pass only through the lower part of the disc stack, since the upper part is blocked with water. Thus separation will be inefficient as only the part of the disc stack will be utilized.
- 90. USE OF CENTRIFUGES (CONTD. – 2) • In order to achieve optimum separation results with purifiers, the interface between oil and water in the bowl must be outside the disc stack but at the inside of the outer edge of the top disc. • To ensure optimal cleaning of a fuel oil, a second separator can be used in series operation, e.g. a purifier followed by a clarifier. The density limit of 991 kg/m3 is not applicable to clarifier operation, but the combined system of purifier and clarifier in series remains restricted to a maximum density of 991kg/m3 at 15°C. • Heavy movements of the vessel can stir up dirt, water and sludge that have accumulated over time on the bottom of the bunker and settling tanks. It is therefore potentially possible that efficient purification is not always obtained when separators have been put in a parallel purifying function.
- 91. CONVENTIONAL CLARIFIER • In a conventional clarifier the water outlet is closed off and the separated water can only be discharged with the sludge through the sludge ports at the bowl periphery. • A sludge discharge causes turbulence in the bowl and leads to less efficient separation. Consequently, the water handling capability of a conventional clarifier is insufficient for the cleaning of fuel oil if the fuel oil has a significant amount of water (the prior use of a purifier with its continuous water removal is mandatory).
- 92. CONVENTIONAL CLARIFIER (CONTD. – 1)
- 93. ADVANCED COMPUTER DRIVEN FUEL CLEANING SYSTEMS • Fuel oils with densities above 991 kg/m3 at 15°C are available in the market and can be purified, with the ALCAP system, which allows fuel oil densities up to 1010 kg/m3 at 15°C. • Fuel oil is continuously fed to the separator. The oil flow is not interrupted when sludge is discharged. The ALCAP basically operates as a clarifier. Clean oil is continuously discharged from the clean oil outlet. Separated sludge and water accumulate at the periphery of the bowl. • Sludge and water is discharged after a pre-set time. If separated water approaches the disc stack (before the pre- set time interval between two sludge discharges is reached) some droplets of water start to escape with the cleaned oil.
- 94. ALCAP SEPARATOR
- 95. ALCAP SYSTEM (CONTD. - 1) • A water transducer, installed in the clean oil outlet immediately senses the small increase of the water content in the clean oil. The signal from the water transducer is transmitted to a control unit and changes in water content are measured. • Increased water content in the cleaned oil is considered as the sign of reduced separation efficiency for not only water but for the removal of solid particles as well. When the water content in the cleaned oil reaches the pre-set trigger point, the control unit will initiate an automatic discharge of the water that has accumulated in the bowl through the water drain valve. That means water is discharged either with the sludge at the periphery of the bowl or through the water drain valve when separated water reaches the disc stack before the pre-set time between sludge discharges.
- 96. RESIDUAL FUELS • This oil is called residual fuel oil (RFO) or heavy fuel oil (HFO). • It is the remainder of the crude oil after gasoline and distillate fuel oils have been extracted through distillation. It fuels thermal power stations or robust engines( marine engines.) • Residual means the material remaining after the removal of more valuable products. • The residue may contain various undesirable impurities including 2 percent water. • Residual fuels are dense, viscous require heating, contain impurities which require treatment before use & have combustion related problems.
- 97. • Residual fuels require preheating from 105 – 145°C for proper atomization at the burners depending on required viscosity. • Fuels used on ship are typically called Bunker Oil which in the present day may be obtained from the heavy gas oil . • or it may be a blend of residual oil with distillate oil to adjust the viscosity. • Residual fuel oil is cheaper
- 98. RESIDUAL FUELS (CONTD. -1) • The most common residual marine fuels are RMG and RMK. The differences between the two are mainly the density and viscosity, with RMG generally being delivered at 380 centistokes or less, and RMK at 700 centistokes or less. Ships with more advanced engines can process heavier, more viscous, and thus cheaper, fuel. • Governing bodies like California, European Union etc. have started to put the limit on the maximum sulphur of fuels burned in their ports to limit pollution. In such places, residual fuels can not be used as they have sulphur contents more than the specified limits, thus Marine Distillate Fuels of grades DMA and DMB are generally in use.
- 99. RESIDUAL FUELS (CONTD. -2) • Main drawback of residual fuel oil is its high initial viscosity which requires a system for storage, heating, pumping, and burning. • Though it is usually lighter than water, it is much heavier and more viscous than distillate fuels. • RMG oil must, in fact, be stored at around 38 °C and heated to 66°C before it can be easily pump. • At cold temperatures it can become a tarry semisolid. The flash point of RMG oil is about 66 °C. • Attempting to pump high-viscosity oil at low temperature can cause the damage to fuel lines, furnaces, and related equipment which are often designed with lighter fuels in mind.
- 100. • The high sulphur content of RMG oil up to 4.5% by weight cause environmental pollution. • When spilled into the sea, this oil does not degrade rapidly. • It’s high viscosity and stickiness is responsible for forming a layer on sea bed.
- 101. EMULSIFIED OILS • Emulsified Fuels are emulsions composed of water and fuel. Emulsion fuels can be either a micro-emulsion or as macro-emulsion. • The essential differences between the two are their stability. Microemulsions are thermodynamically stable whereas macroemulsions are kinetically stabilized. • Microemulsions are formed spontaneously whereas macroemulsions are formed by a shearing process. • Particle size distribution in micro-emuslion have dimensions of 10 to 200 nm and in macroemulsion have 100 nm to over 1 micrometer. • Micro-emulsions are isotropic whereas macro-emulsions are prone to settling and changes in particle size over time.
- 102. EMULSIFIED OILS (CONTD. – 1) • Water-in-oil emulsified fuels contain between 5 and 30% water (by mass) in the overall fuel emulsion. • The main advantages of using emulsified fuels instead of plain fuel are environmental protection and economic benefits. Addition of water to the diesel decreases combustion temperatures and lowers NOx emissions. • Usual method is to inject water along with fuel although some engine builders prefer emulsified fuels as it further helps in atomization of fuel. Fuel is first atomized in the fuel valve by passing it at high pressure through fine orifices and secondly when water droplet explodes in combustion chamber on receiving the heat gives secondary atomization.
- 103. ADDITIONAL QUESTION AND ANSWER • ABSOLUTE VISCOSITY • The resistance to flow encountered when one layer or plane of fluid attempts to move over another identical layer or plane of fluid at a given speed. Absolute viscosity is also called dynamic viscosity.
- 104. • Pour point: • The pour point is the temperature below which the fuel ceases to flow. Once the fuel oil temperature goes below the pour point, it forms wax which can lead to blockage of the filter. The wax formation will also build upon tank bottoms and heating coils, leading to a reduction in heat exchanging capabilities.
- 105. • Sulphur: • Sulphur in the fuel is one of the main factors for sulphur oxide pollution from ships – a pollutant which is currently under major scrutiny. As per MARPOL, the current sulphur value for HFO are: • 3.50% m/m on and after 1 January 2012 • 0.50% m/m on and after 1 January 2020
- 106. ASH • The amount of inorganic materials present in the fuel which remain as residue once the combustion process is over is called ash deposits. These deposits mainly consist of elements such as vanadium, sulphur, nickel, sodium, silicon, aluminium etc., which are already present in the fuel. • The max. limit of ash content in the fuel is 0.2% m/m.
- 107. LNG • Liquefied natural gas (LNG) is natural gas that has been cooled to a liquid state, at about - 260°Fahrenheit, for shipping and storage. The volume of natural gas in its liquid state is about 600 times smaller than its volume in its gaseous state.
- 108. LNG • Is natural gas (predominantly methane, CH4, with some mixture of ethane C2H6) that has been cooled down to liquid form for ease and safety of non-pressurized storage or transport. • It takes up about 1/600th the volume of natural gas in the gaseous state (at standard conditions for temperature and pressure). • It is odorless, colorless, non-toxic and non- corrosive.
- 109. • Hazards include flammability after vaporization into a gaseous state, freezing and asphyxia. • The liquefaction process involves removal of certain components, such as dust, acid gases, helium, water, and heavy hydrocarbons, which could cause difficulty downstream.
- 110. ADVANTAGES • ECONOMY CHEAPER THAN ANY OTHER FOSSIL FUEL. • ENVIORNMENT Environment: Another important fact is that natural gas burns without releasing any soot or sulfur dioxide. It also emits 45% less carbon dioxide than coal and 30% less than oil.
- 111. • Transportation: Transportation is made via sea (tankers) and land (pipelines and small tanks). This fact allows natural gas to be easily transferred from power plants to residential areas. Multi-uses: Natural gas is a multi-use fuel. It can be used for generating electric power, powering Ships (by substituting for diesel and gasoline), •
- 112. • Availability: It is abundant and almost worldwide available. • Conversion to Hydrogen Fuel: It is currently the cheapest fossil fuel source for producing hydrogen.
- 113. DISADVANTAGES • Flammable and Toxic: Natural gas leaks can be proven to be extremely dangerous. Such leaks may be the cause of fire or explosions. The gas itself is extremely toxic when inhaled. • Non-Renewable: It is a finite source of energy and cannot be considered a long-term solution to our energy supply problem.
- 114. • Processing: In order to use it as a fuel, all constituents other than methane have to be extracted. The processing results in several byproducts: hydrocarbons (ethane, propane, etc.), sulfur, water vapor, carbon dioxide, and even helium and nitrogen. • Installation: The whole pipe installation may be very expensive to construct since long pipes, specialized tanks.
- 115. • Environmental Impact: When natural gas burns, carbon dioxide, monoxide, and other carbon compounds are emitted in the atmosphere contributing to the greenhouse effect. Although it is cleaner than other fossil fuels (oil, coal, etc.) as far as byproducts are concerned, natural gas leaks can become more hazardous since methane is 21 times more dangerous than carbon dioxide. •
- 116. • The natural gas is then condensed into a liquid at close to atmospheric pressure by cooling it to approximately −162 °C (−260 °F); maximum transport pressure is set at around 25 kPa (4 psi).