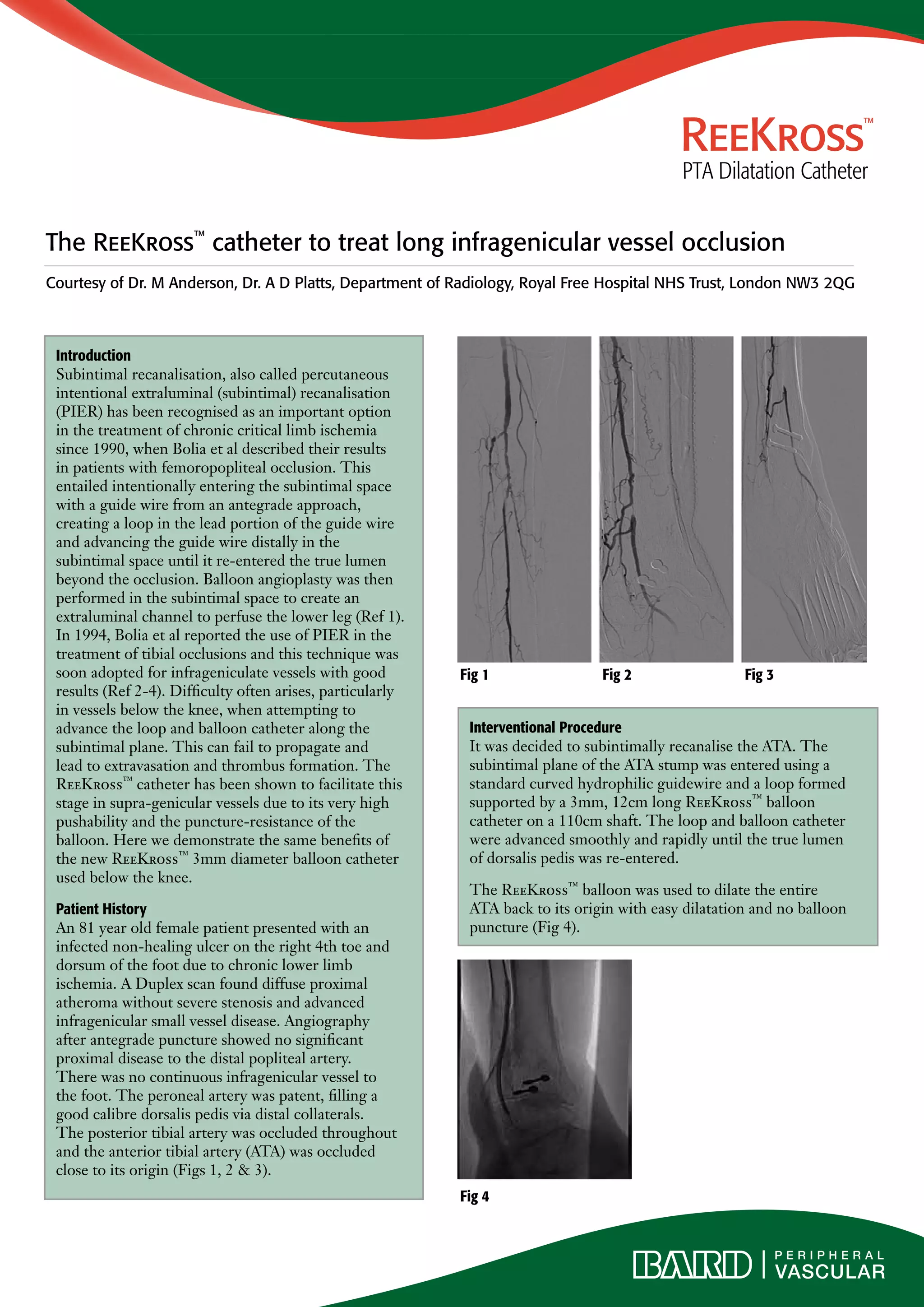
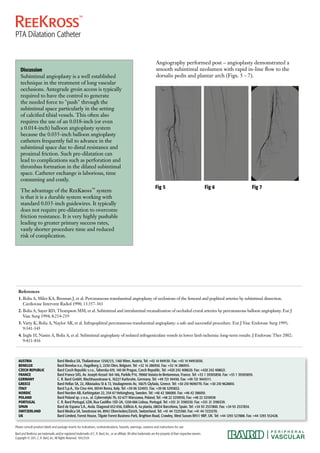
The document describes the use of the ReeKross catheter to treat an occlusion of the anterior tibial artery (ATA) in an 81-year old female patient presenting with an infected non-healing ulcer on her right foot due to chronic lower limb ischemia. The subintimal plane of the occluded ATA was entered and the ReeKross catheter was used to advance smoothly through the subintimal space to reenter the true lumen beyond the occlusion. Balloon angioplasty with the ReeKross catheter then dilated the entire length of the ATA with easy dilatation and no balloon puncture, restoring blood flow to the foot as seen on follow up angiography. The Ree