Introduction to hydrogen fine structure.pdf
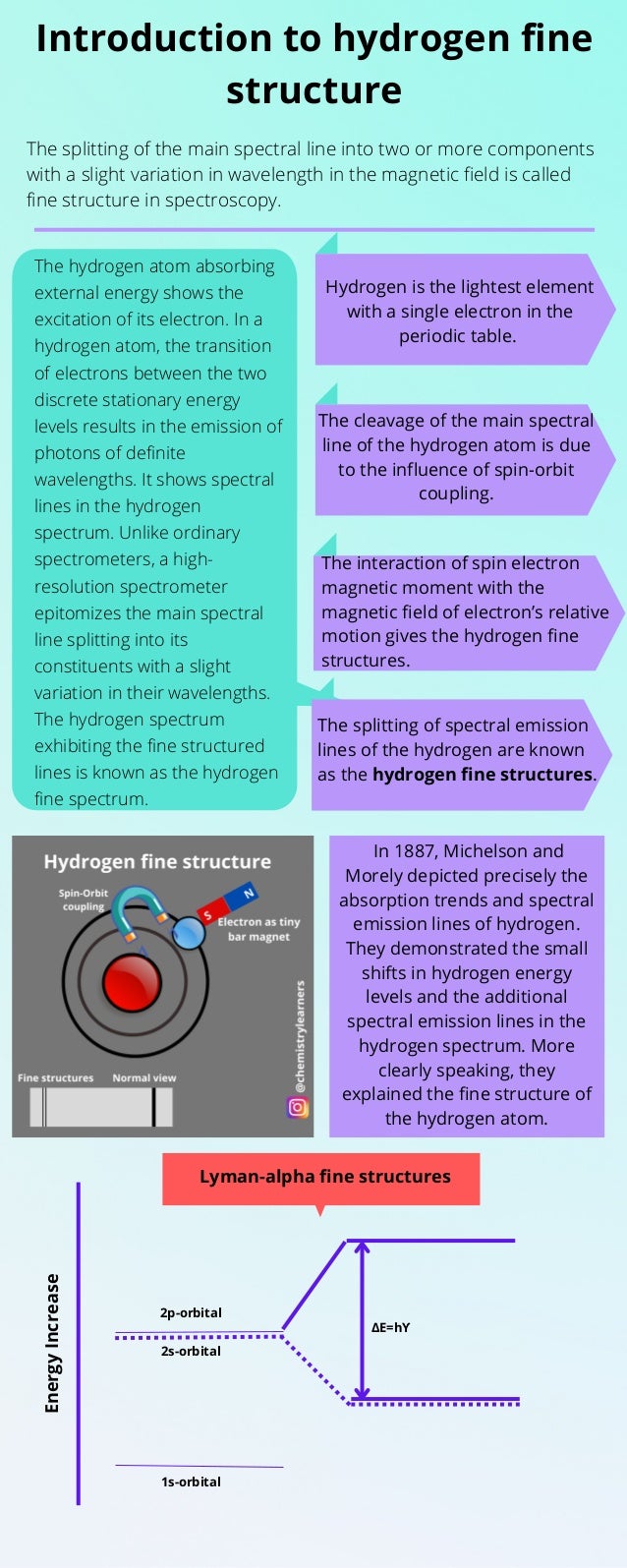
- 1. Introduction to hydrogen fine structure The splitting of the main spectral line into two or more components with a slight variation in wavelength in the magnetic field is called fine structure in spectroscopy. Hydrogen is the lightest element with a single electron in the periodic table. The cleavage of the main spectral line of the hydrogen atom is due to the influence of spin-orbit coupling. The interaction of spin electron magnetic moment with the magnetic field of electron’s relative motion gives the hydrogen fine structures. The splitting of spectral emission lines of the hydrogen are known as the hydrogen fine structures. The hydrogen atom absorbing external energy shows the excitation of its electron. In a hydrogen atom, the transition of electrons between the two discrete stationary energy levels results in the emission of photons of definite wavelengths. It shows spectral lines in the hydrogen spectrum. Unlike ordinary spectrometers, a high- resolution spectrometer epitomizes the main spectral line splitting into its constituents with a slight variation in their wavelengths. The hydrogen spectrum exhibiting the fine structured lines is known as the hydrogen fine spectrum. In 1887, Michelson and Morely depicted precisely the absorption trends and spectral emission lines of hydrogen. They demonstrated the small shifts in hydrogen energy levels and the additional spectral emission lines in the hydrogen spectrum. More clearly speaking, they explained the fine structure of the hydrogen atom. Energy Increase Lyman-alpha fine structures 1s-orbital 2s-orbital 2p-orbital ΔE=hϒ
- 2. Explanation The transition of an electron from 1S-orbit to 2P-orbit gives a spectral line doublet in the presence of the magnetic field. The Lyman alpha doublet consists of closely spaced two spectral emission lines at wavelengths of about 121.5668 nm and 121.5674 nm. And they are symbolized as Ly-α3/2 and Ly-α1/2 having j values 3/2 and 1/2, where j is the total angular momentum of the electron. The electron motion is associated with the orbital quantum number (l) and the spin quantum number (s). Hence, the total angular moment quantum number (j) can be expressed as j=l+s (or) j=l-s When the electron is in 1S- orbit, its spin quantum number values are +1/2 and -1/2 depending upon the direction of the magnetic moment. Due to the absence of spin-orbit coupling in 1S-orbit, splitting does not take place in electron energy levels. So, the 1S-orbit has a single energy level. In 2P-orbit, the spin-orbit interaction breaks the main energy level into its components. So, we observe two sub-energy levels for the electron in the 2P-orbit. The spin angular momentum quantum number values for the electron are +1/2 and -1/2. Additionally, the orbital angular momentum quantum number value for P-subshell is 1. The total angular momentum quantum number values are 1/2 and 3/2. The hydrogen electron transition from 1S-orbit to 2P-orbit gives spectral lines doublet as it involves the two 2P-orbit energy sub-states in the electron transition. The spectral line doublet is a pair of two closely spaced spectral lines with a slight variation in their wavelengths. Blog: https://jayamchemistrylearners.blogspot.com/ The alkali metal atoms with 1S- electron in their valence shell give spectral line doublet in the presence of the magnetic field.