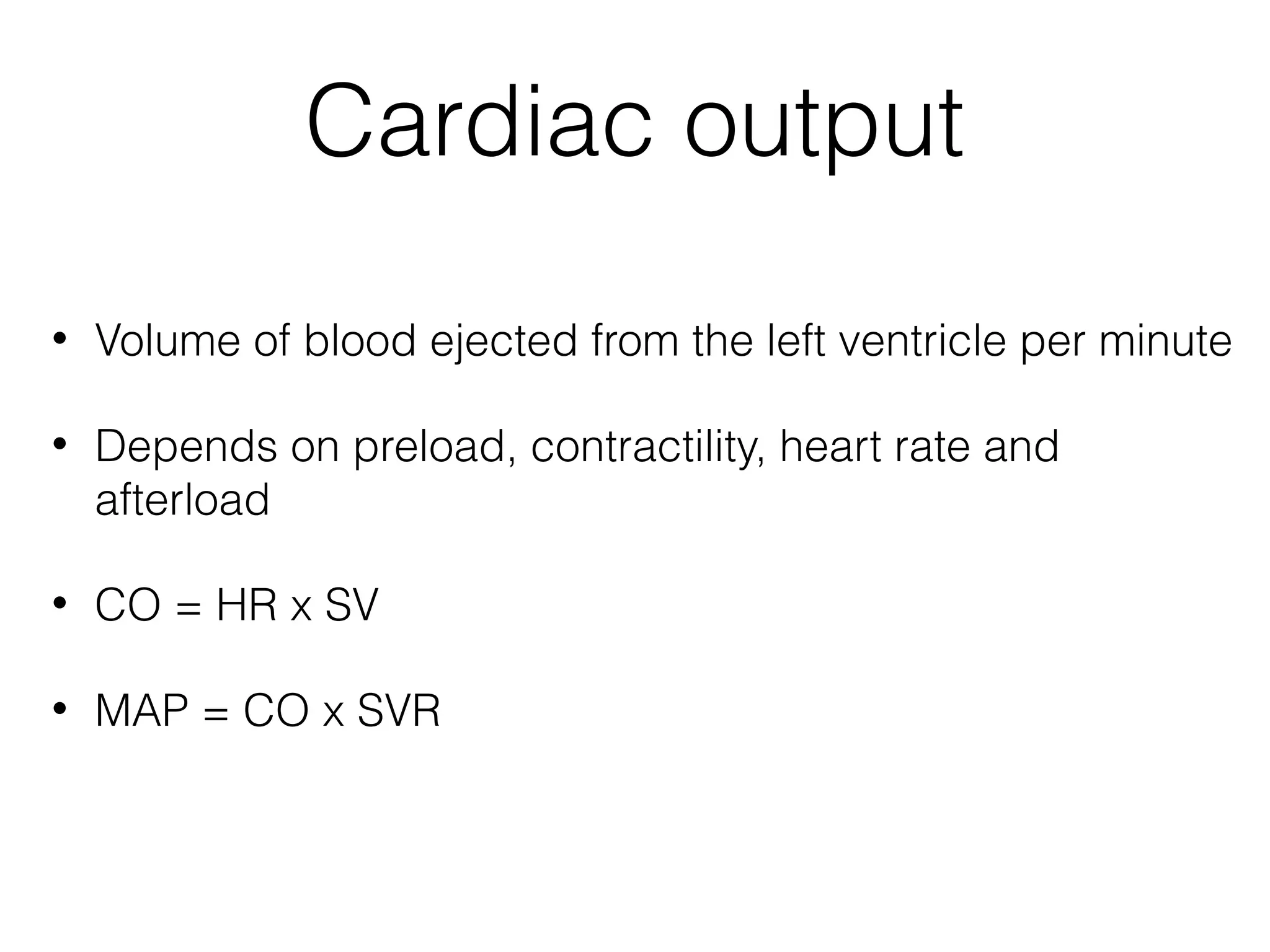
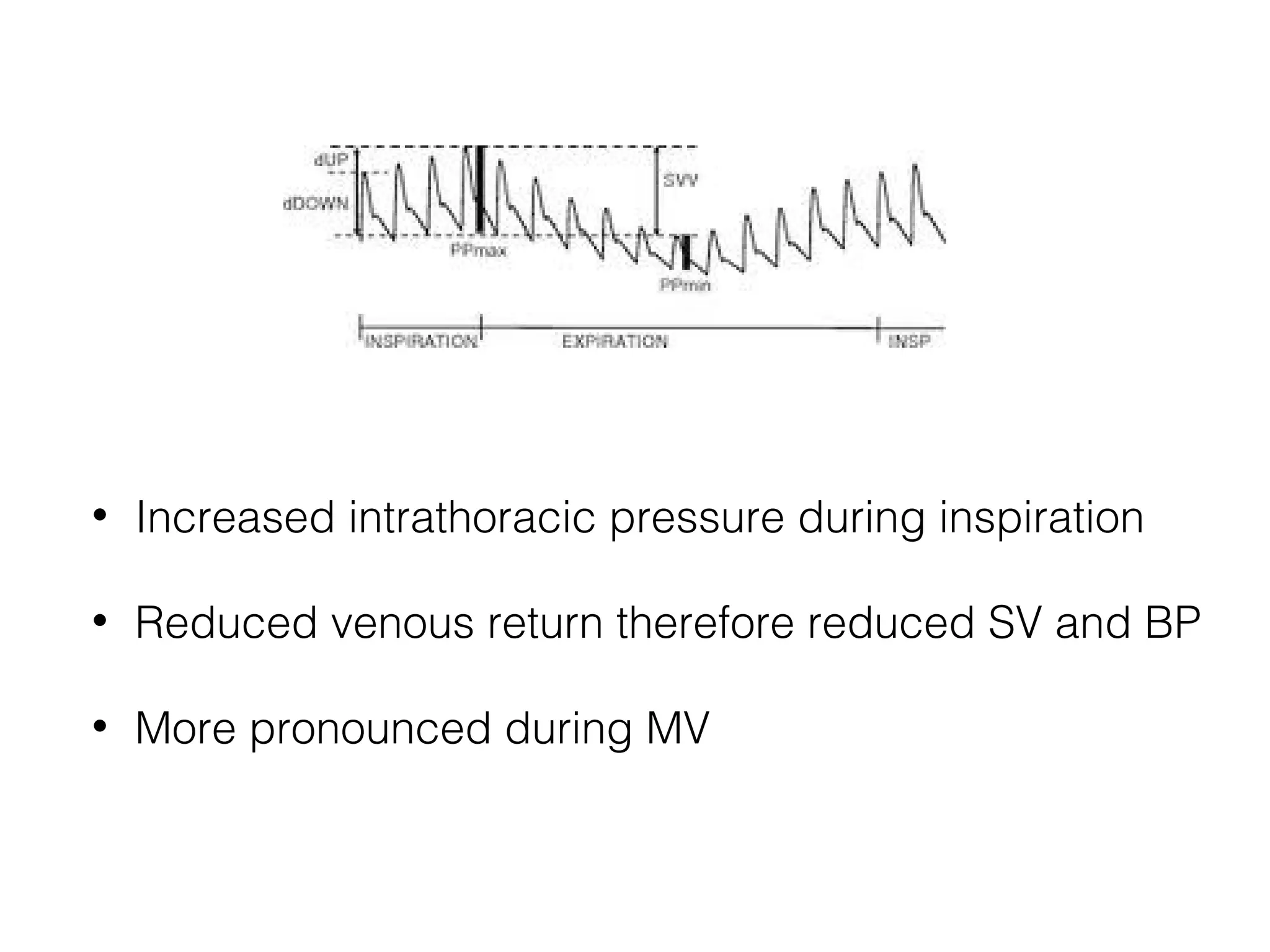
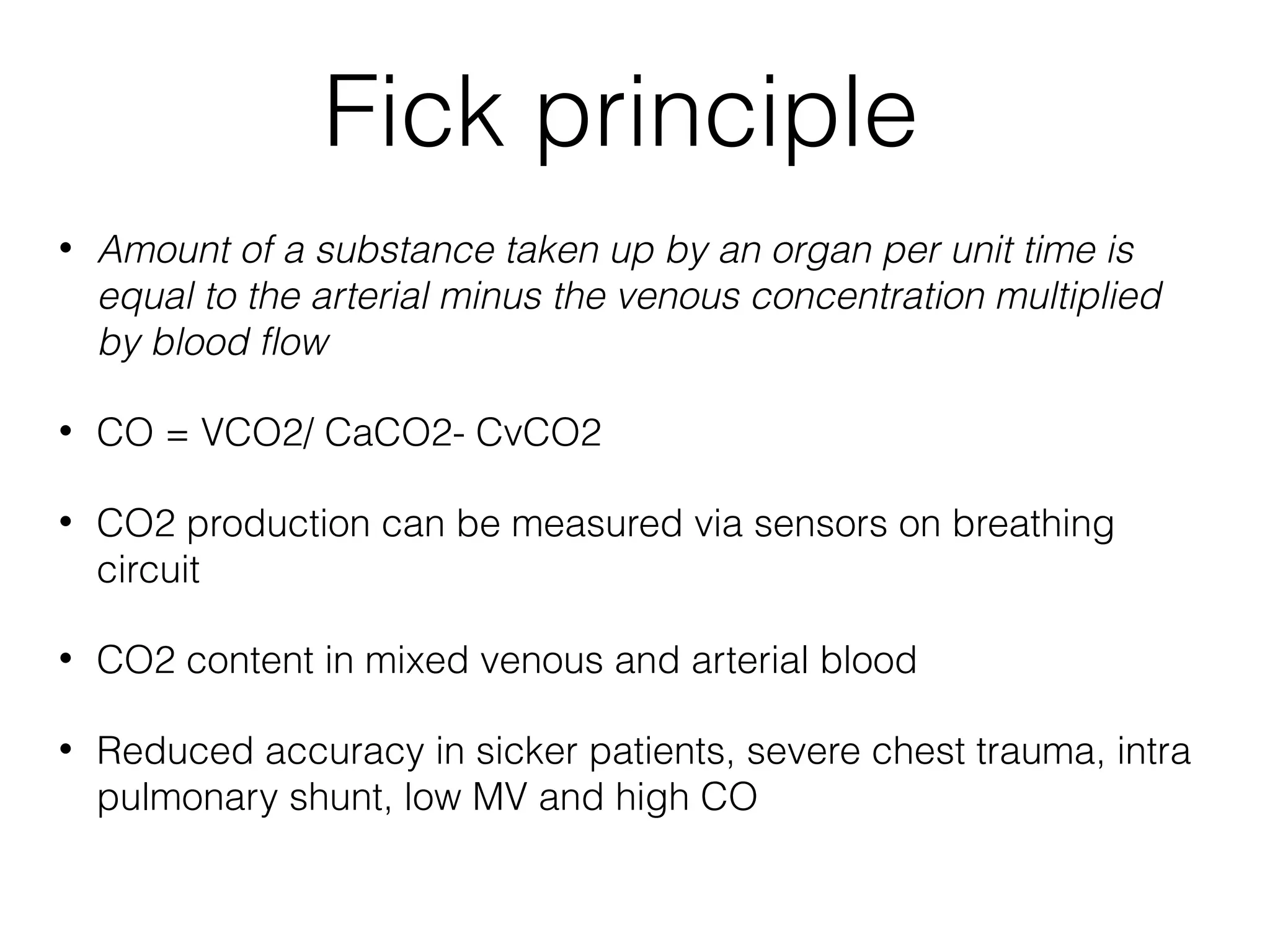
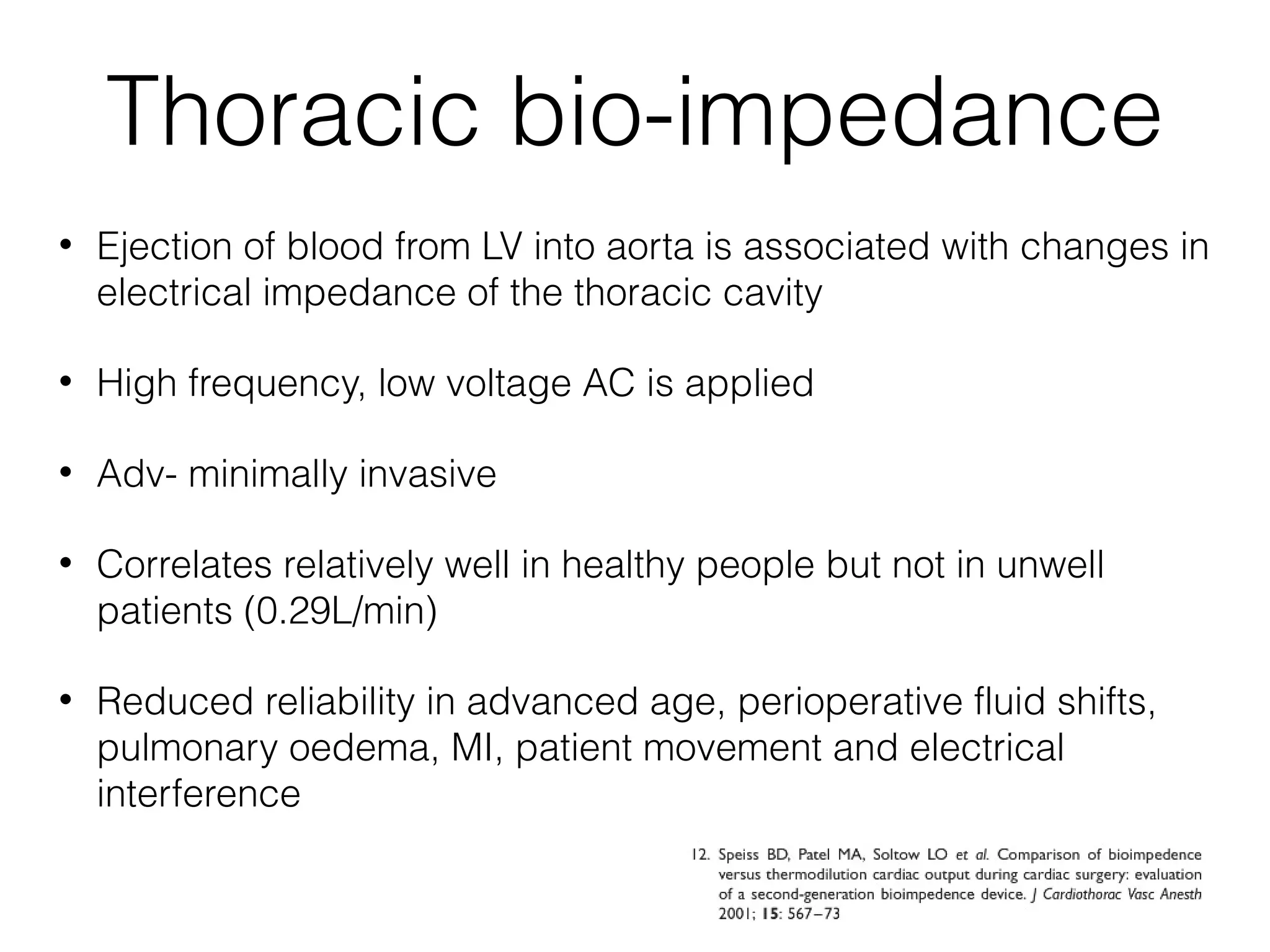
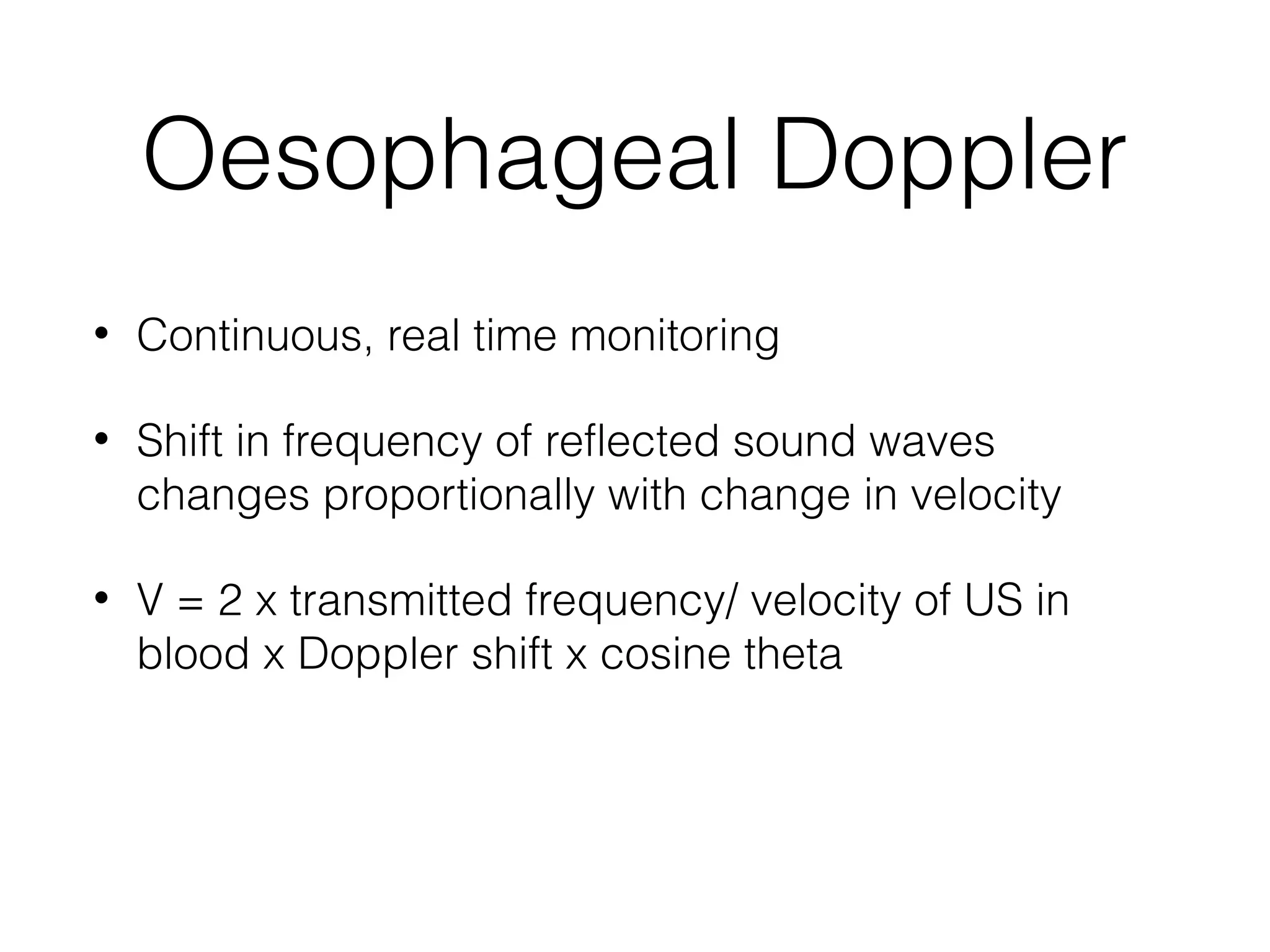
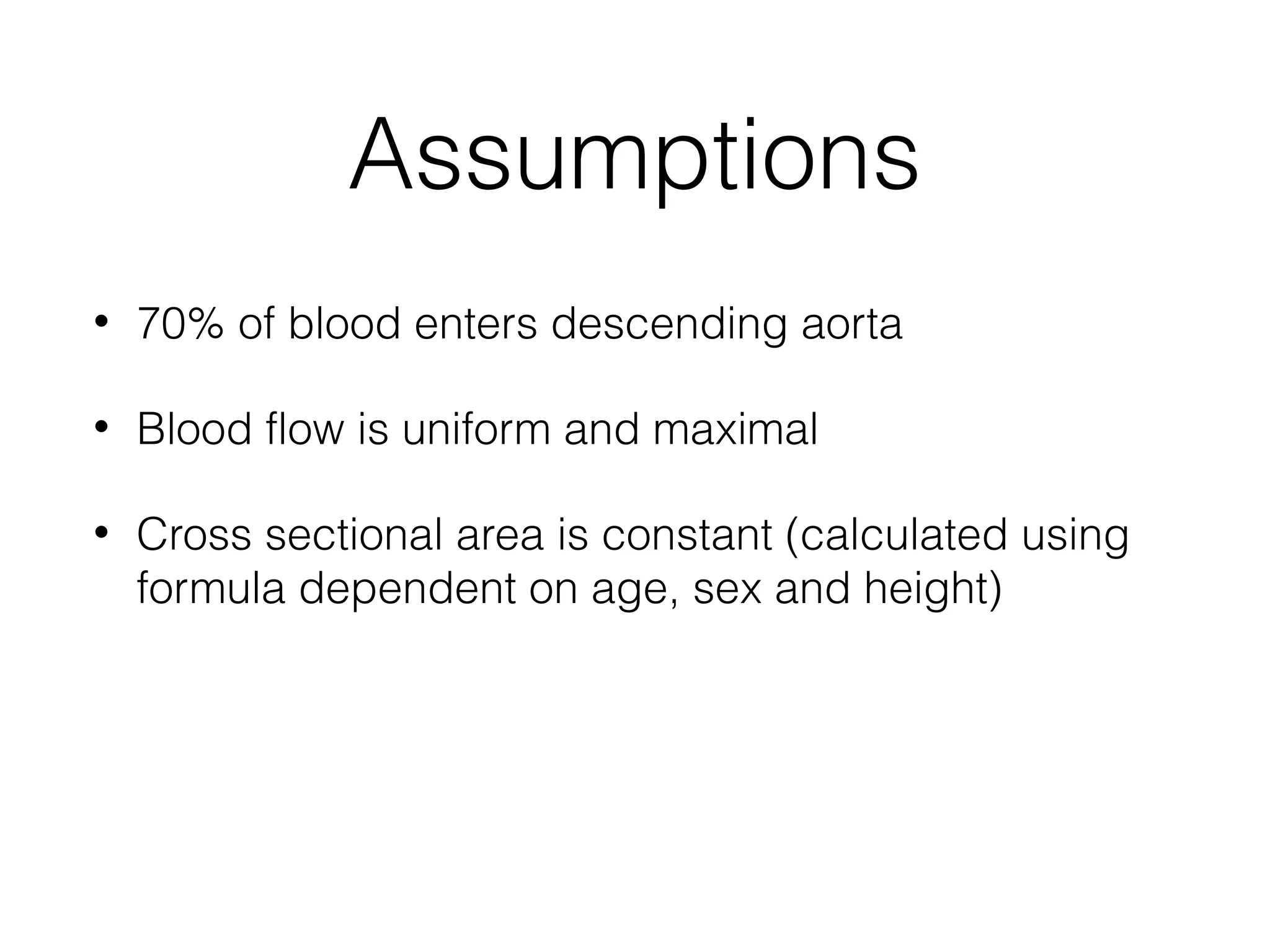
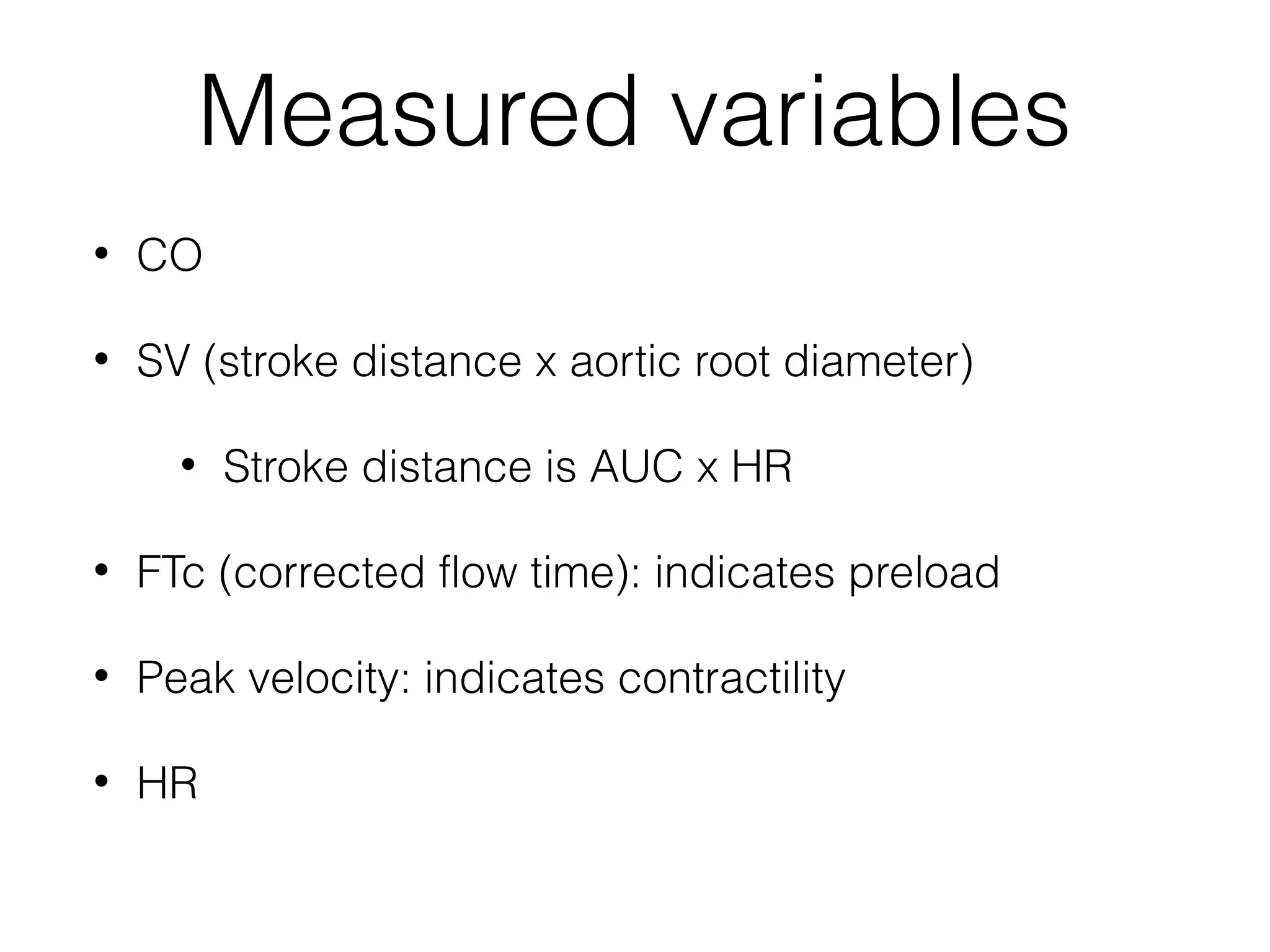
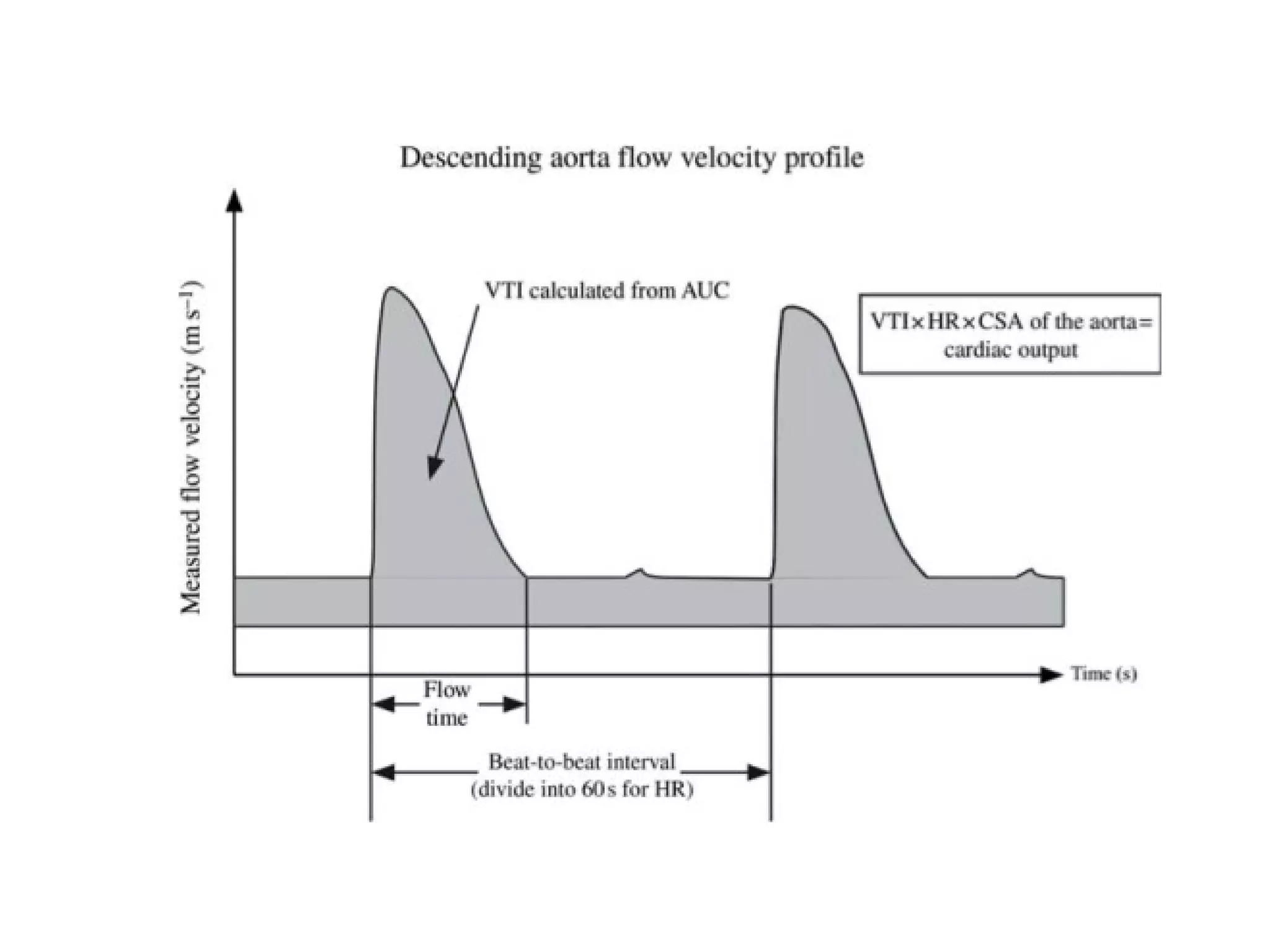
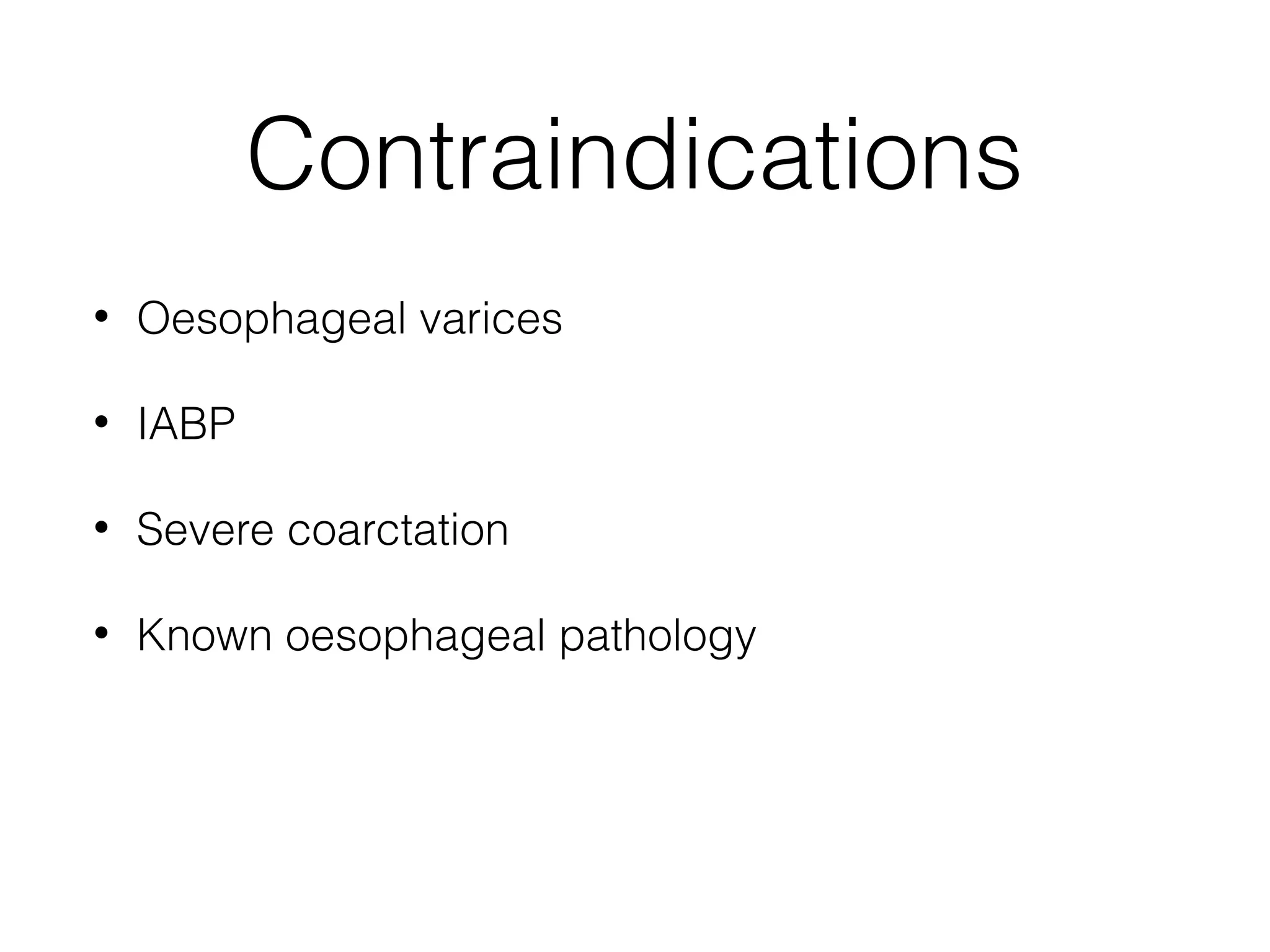
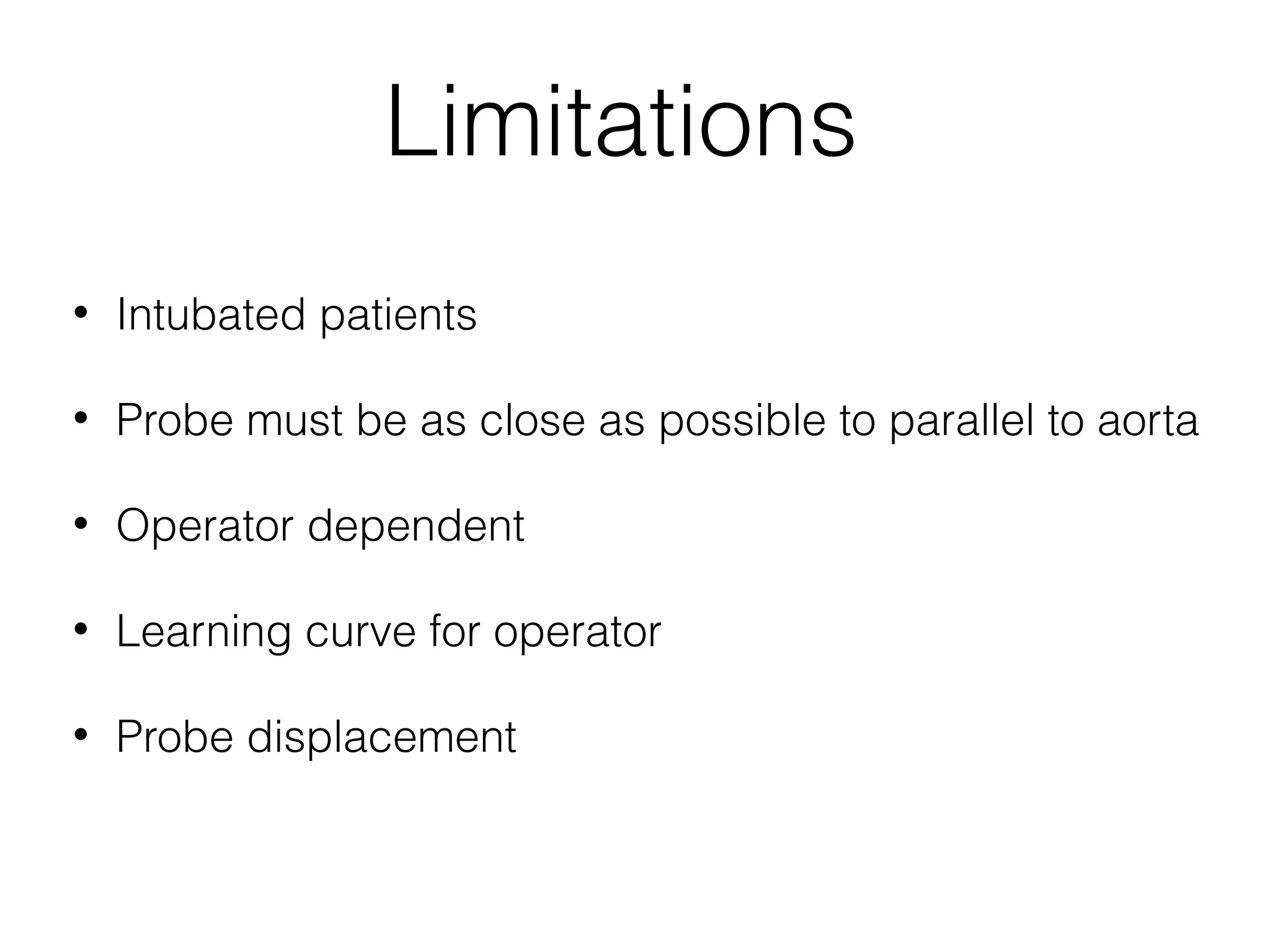
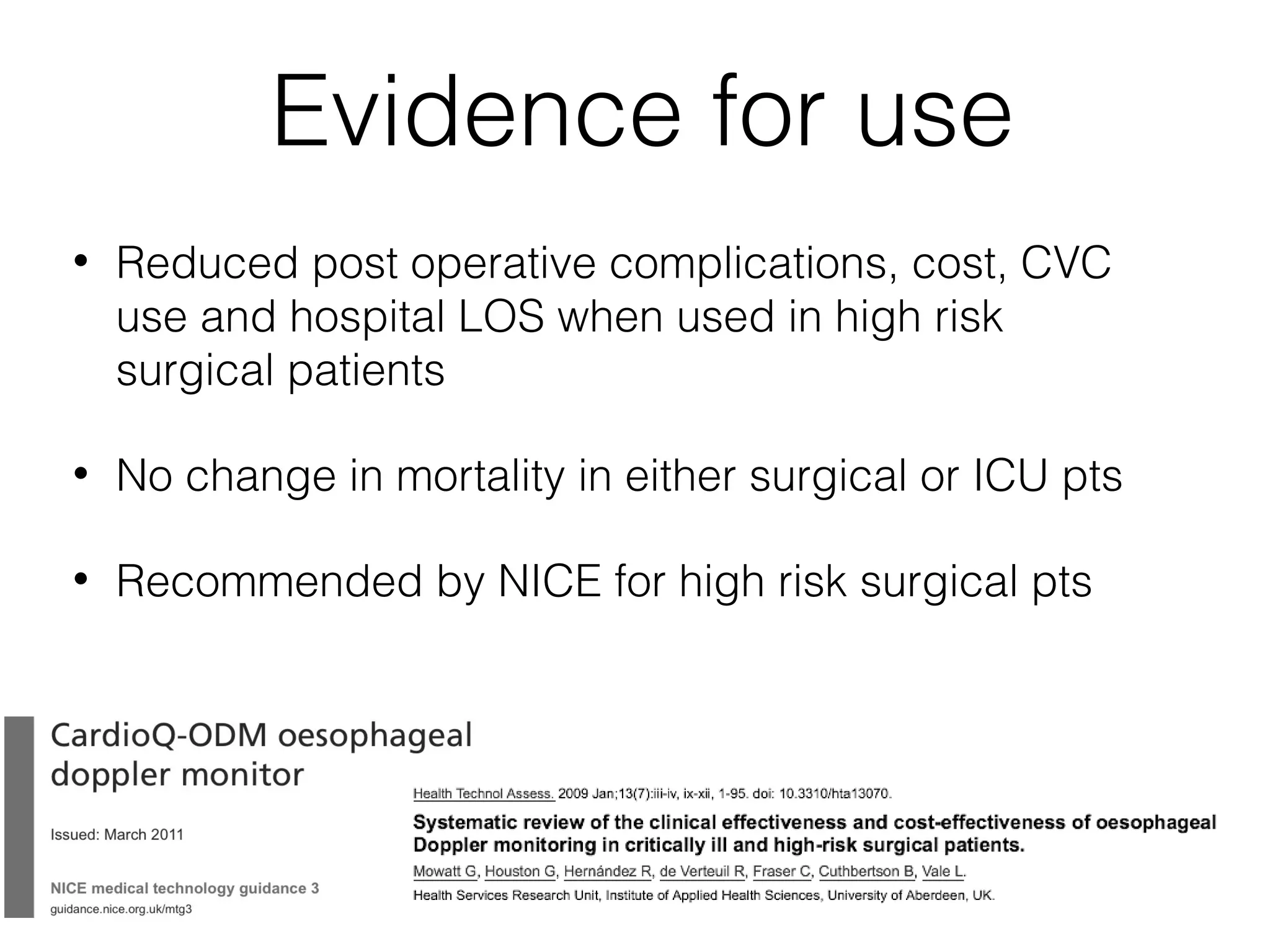
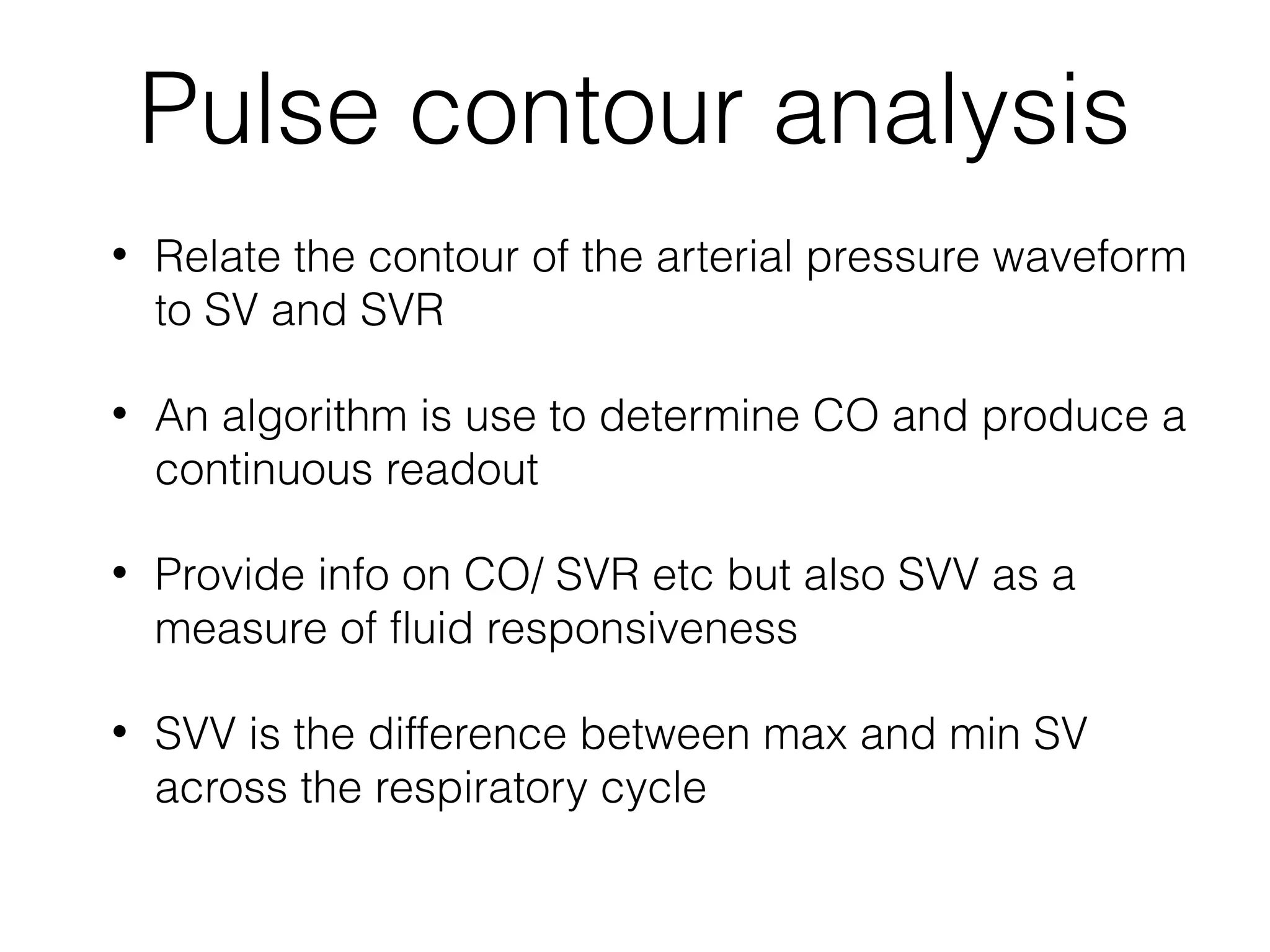
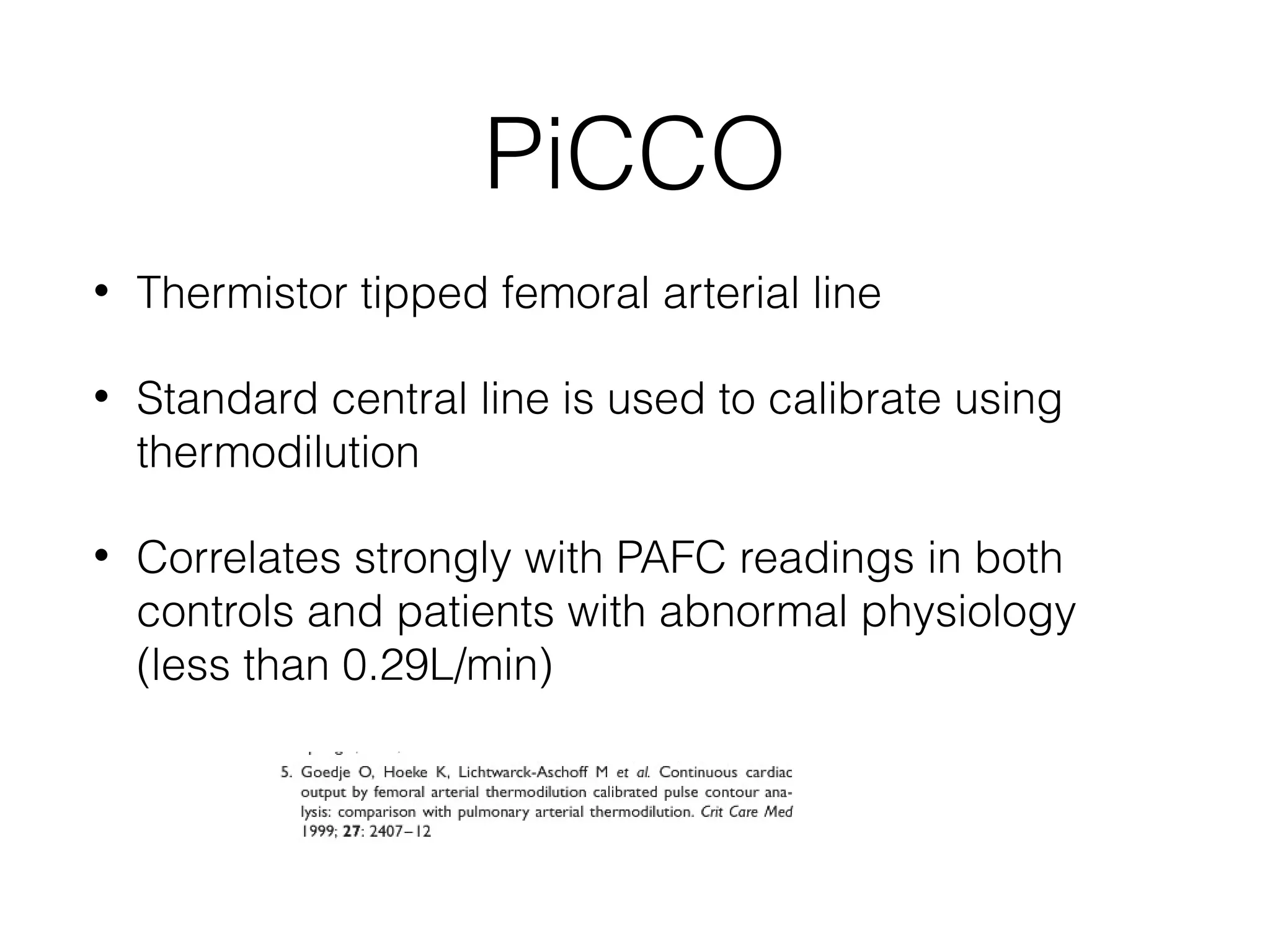
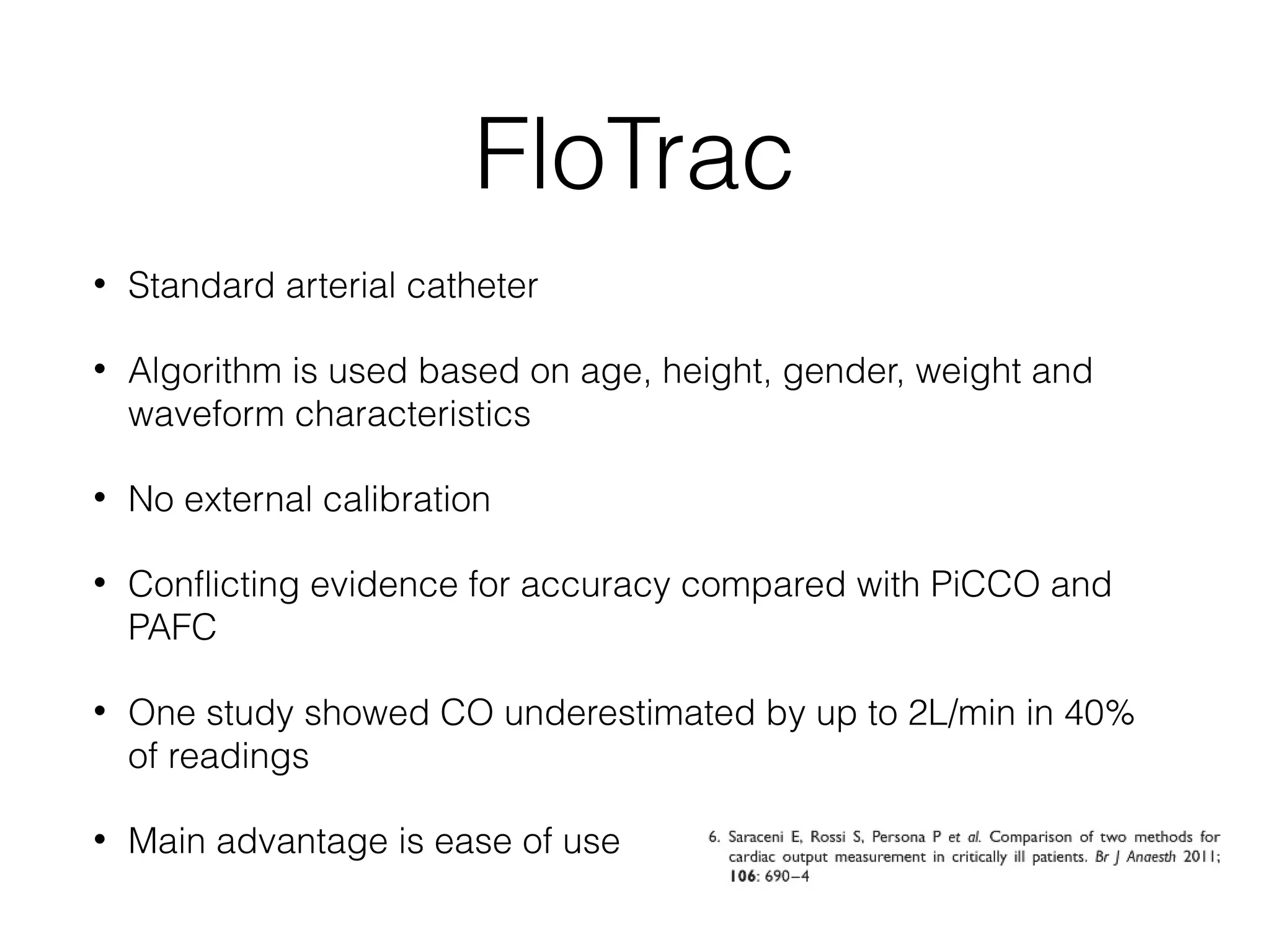
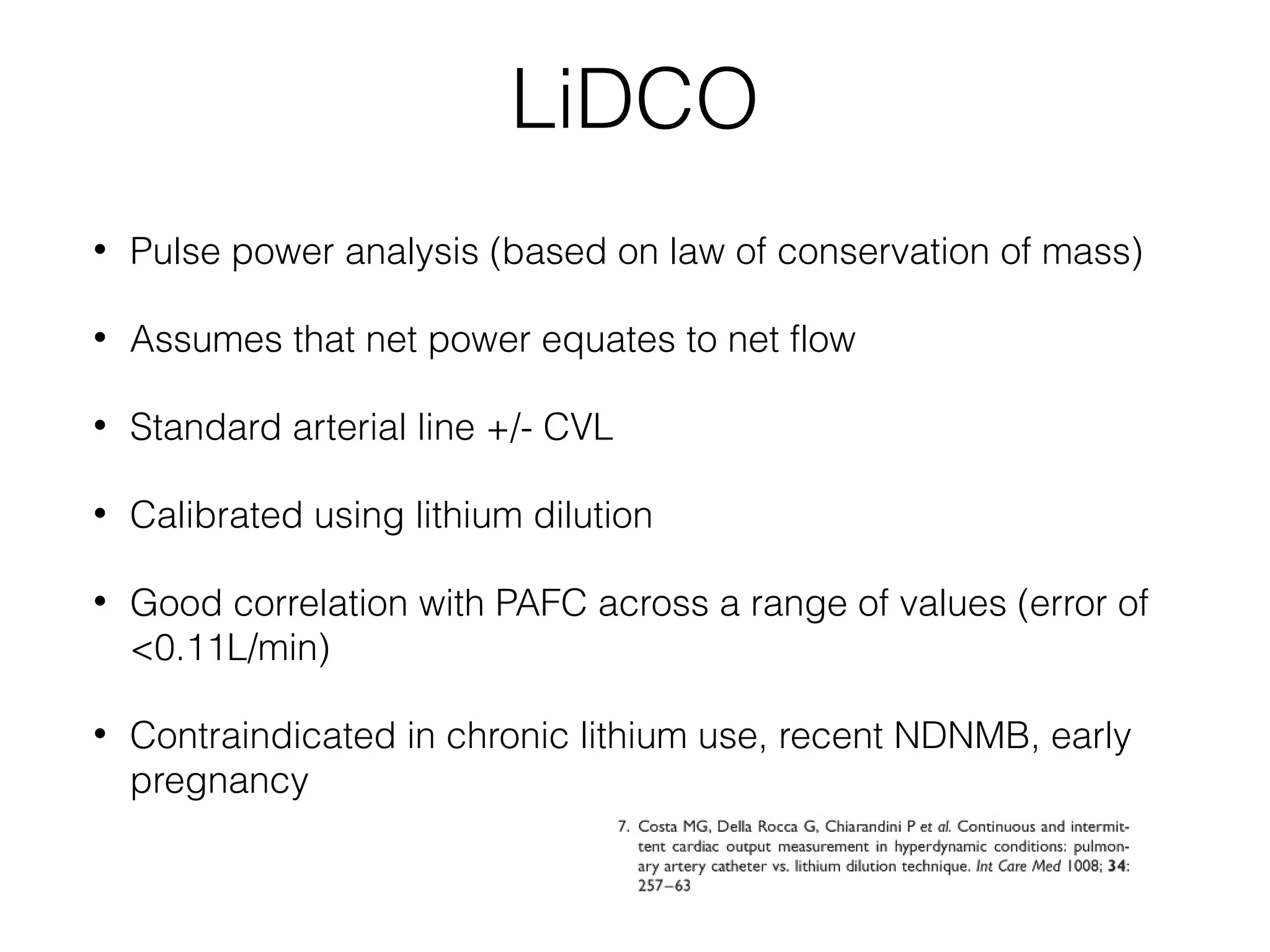
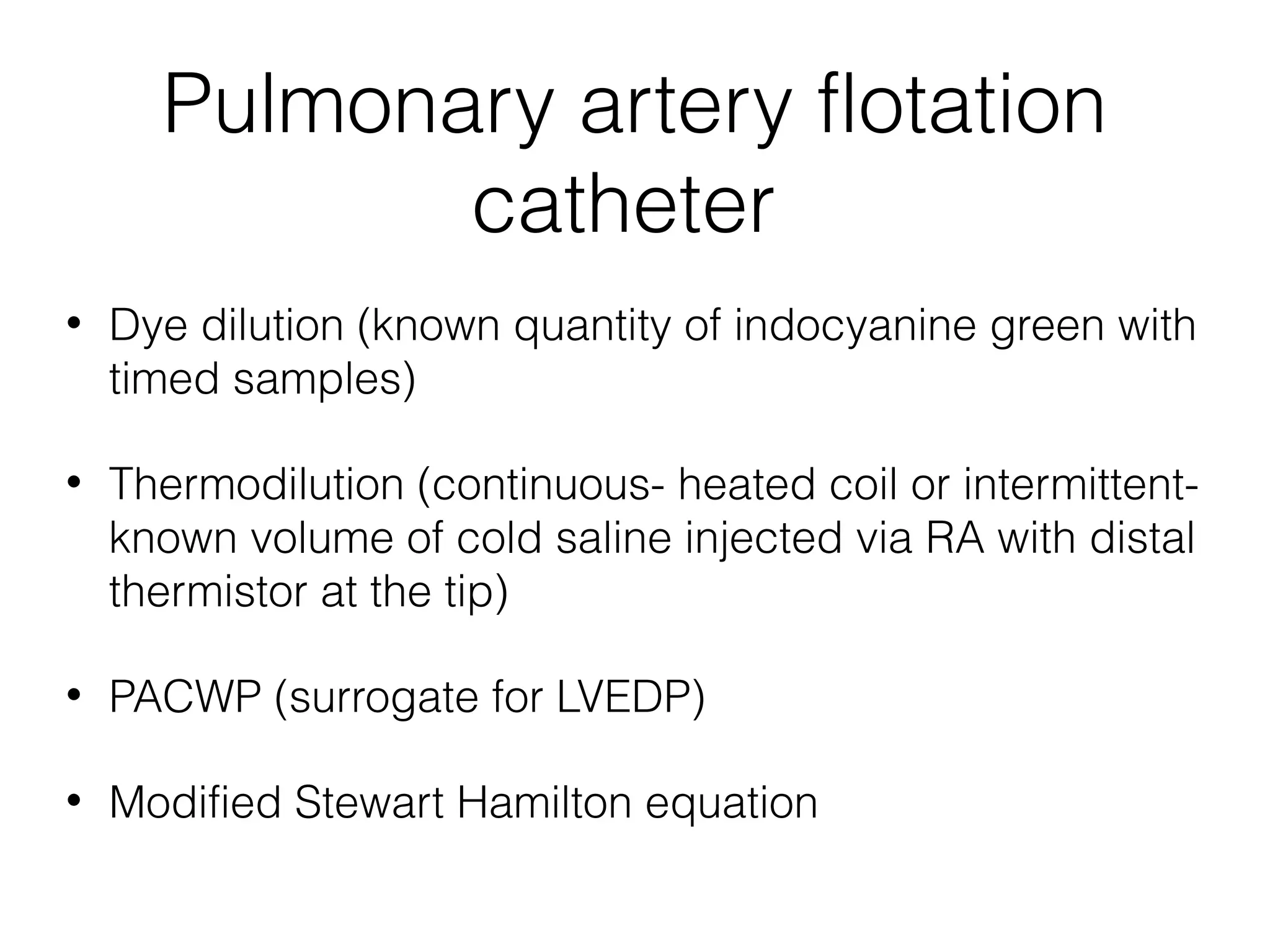
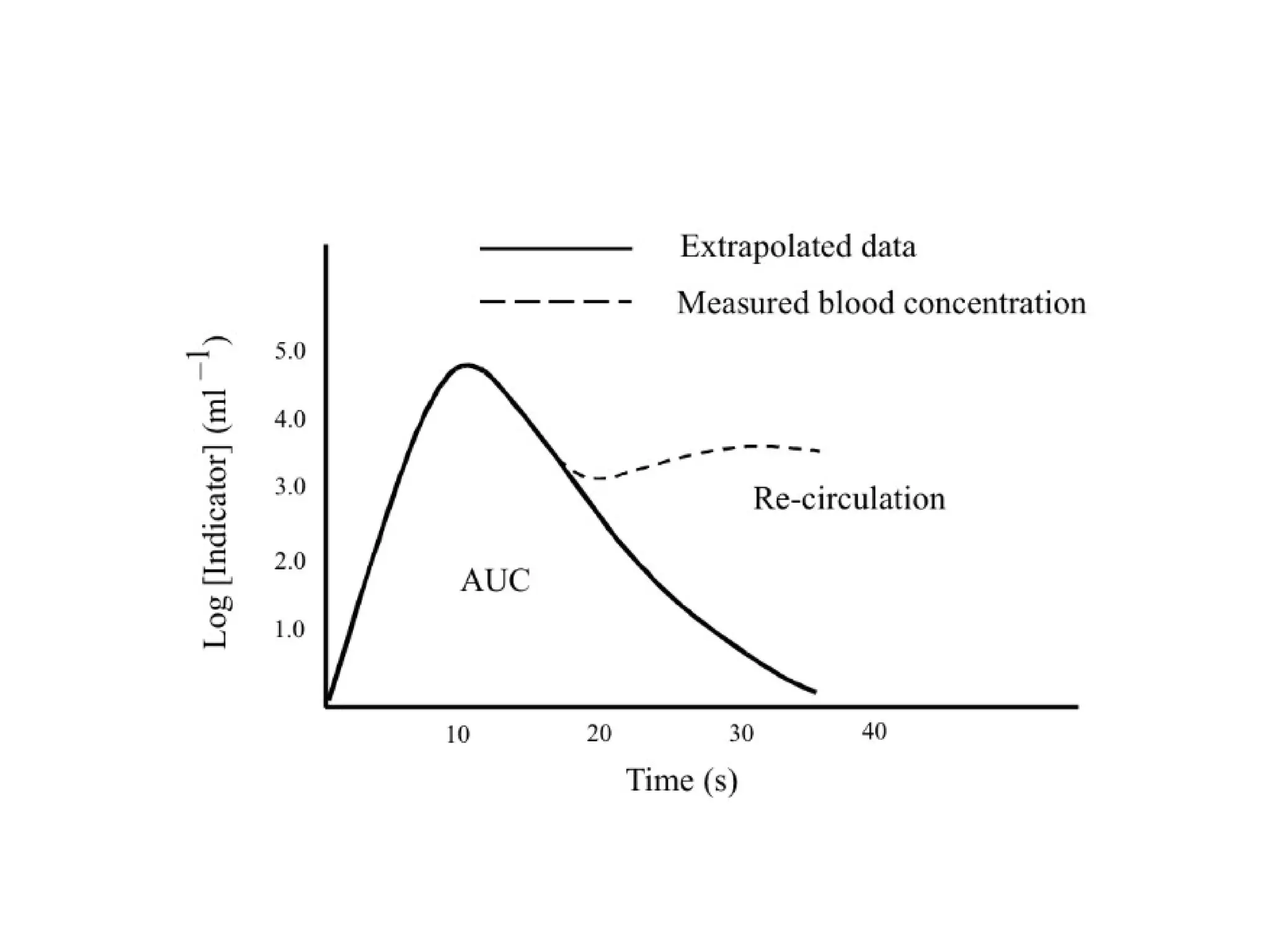
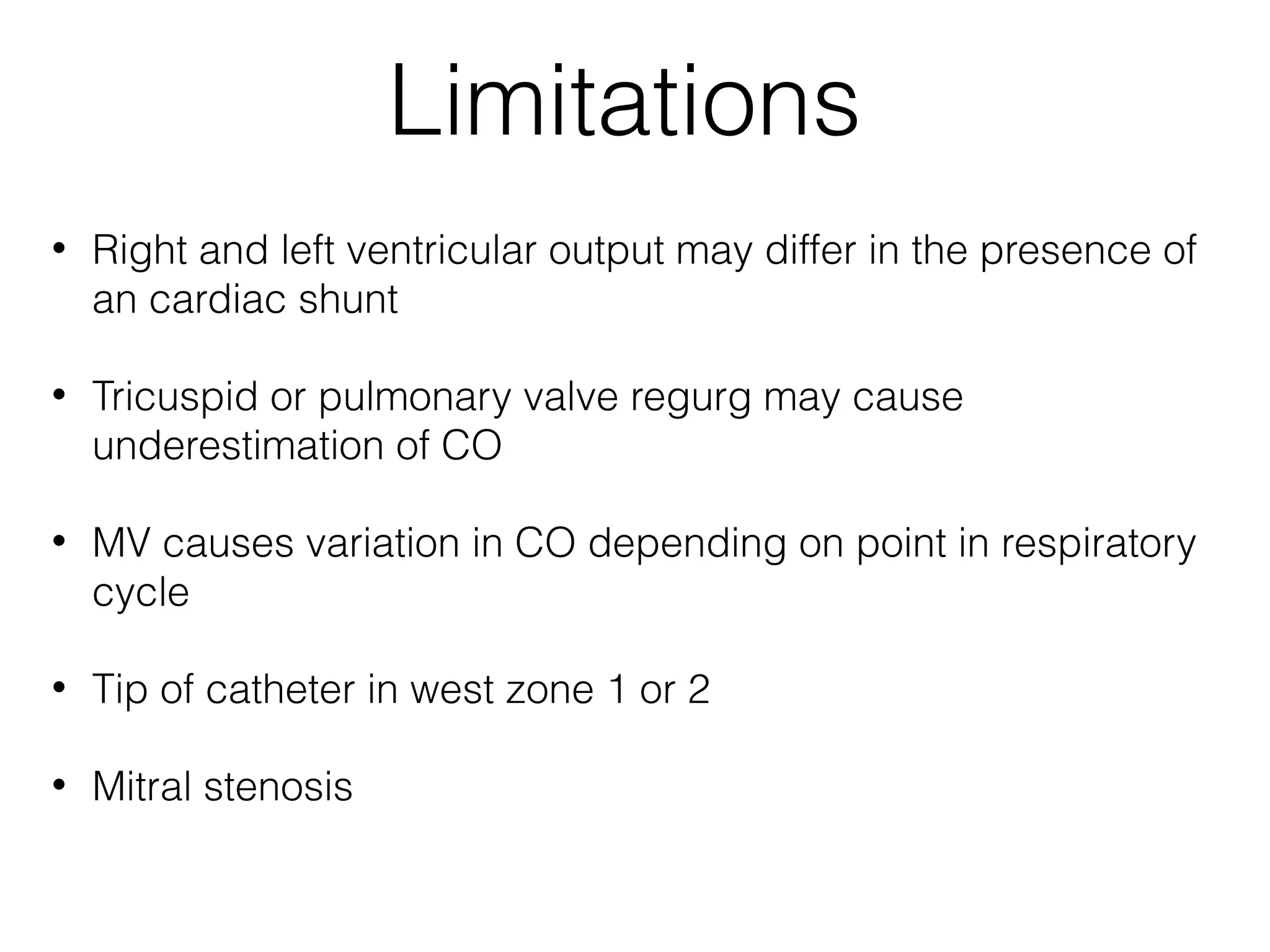
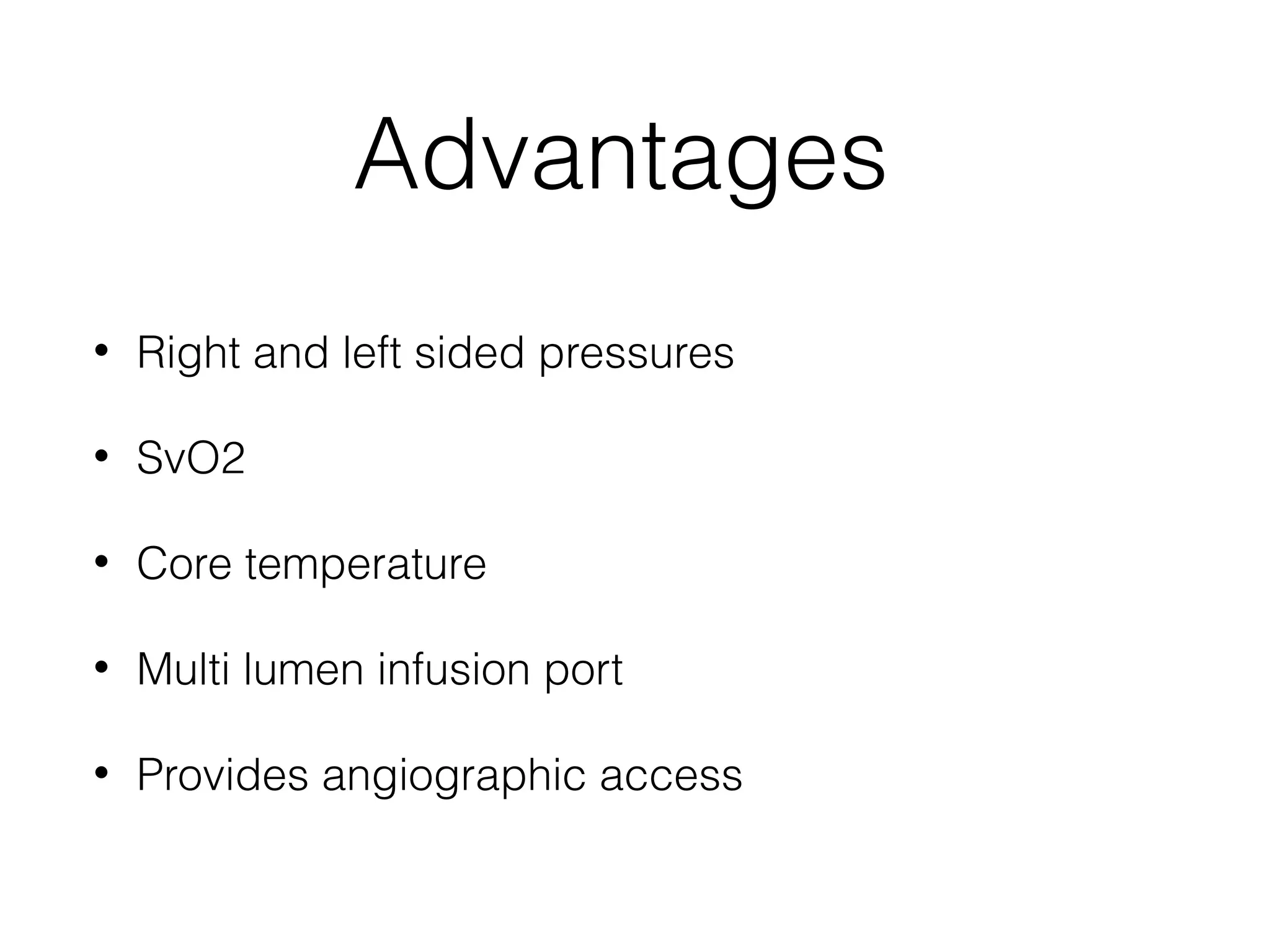
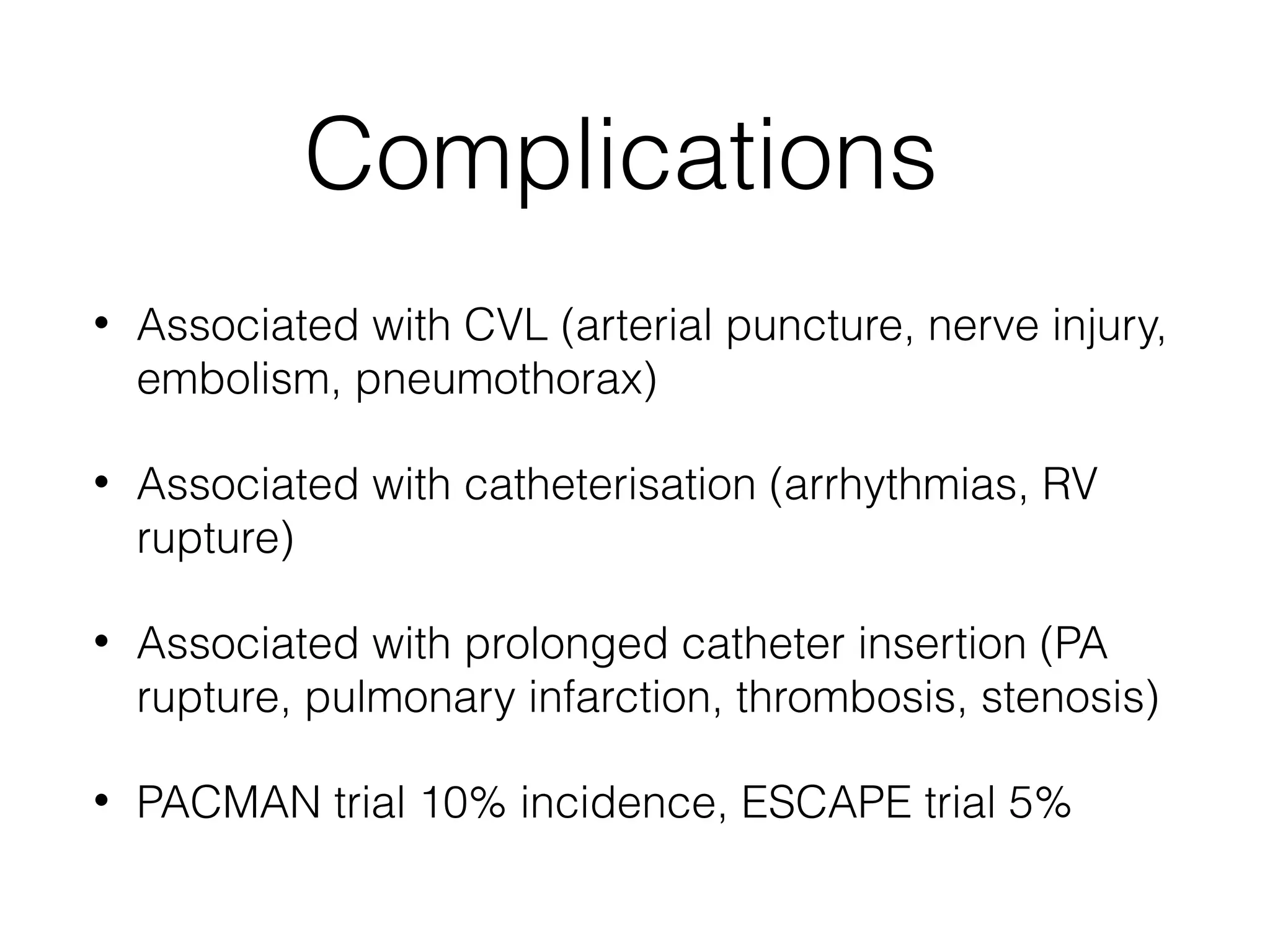
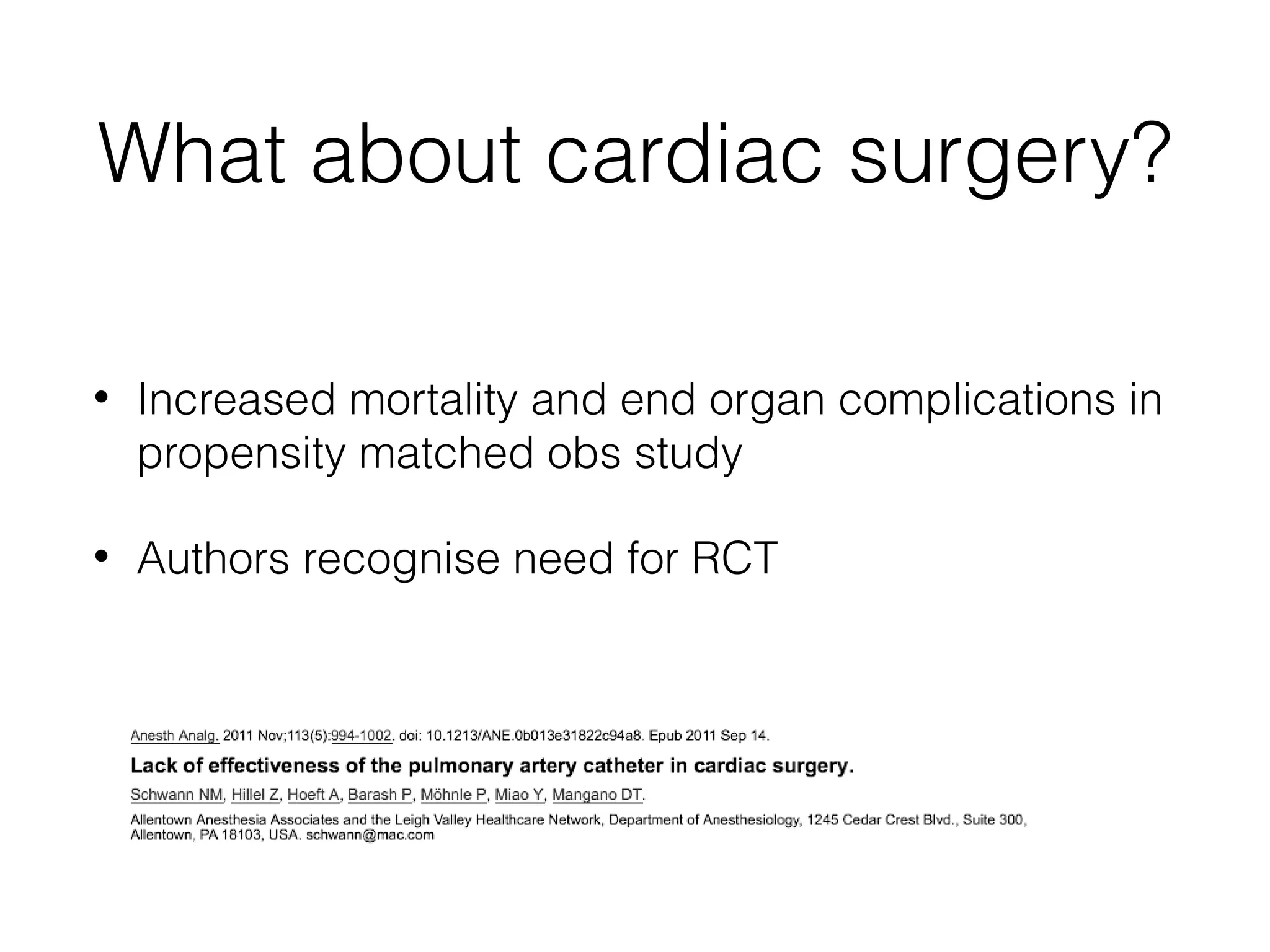
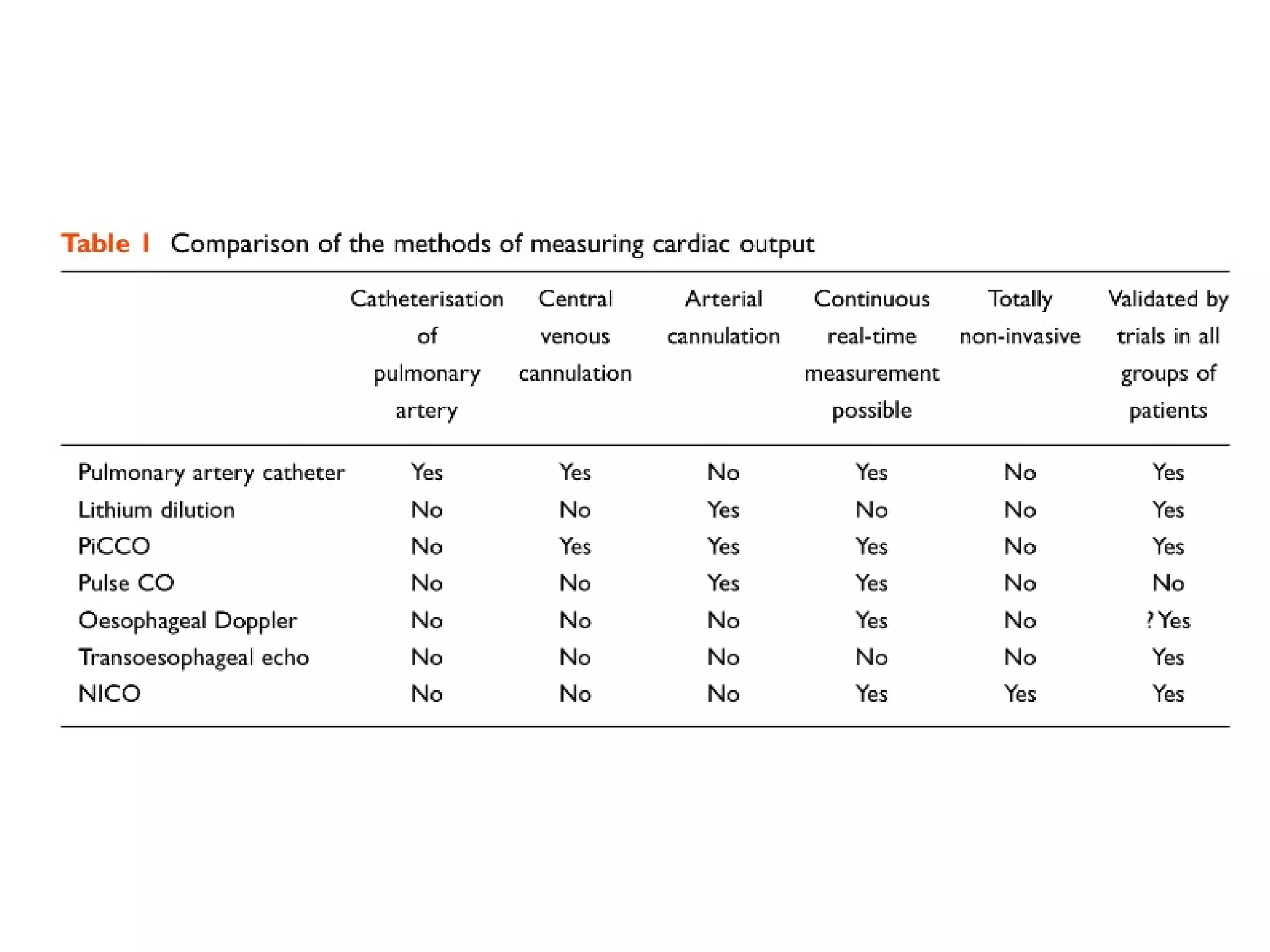
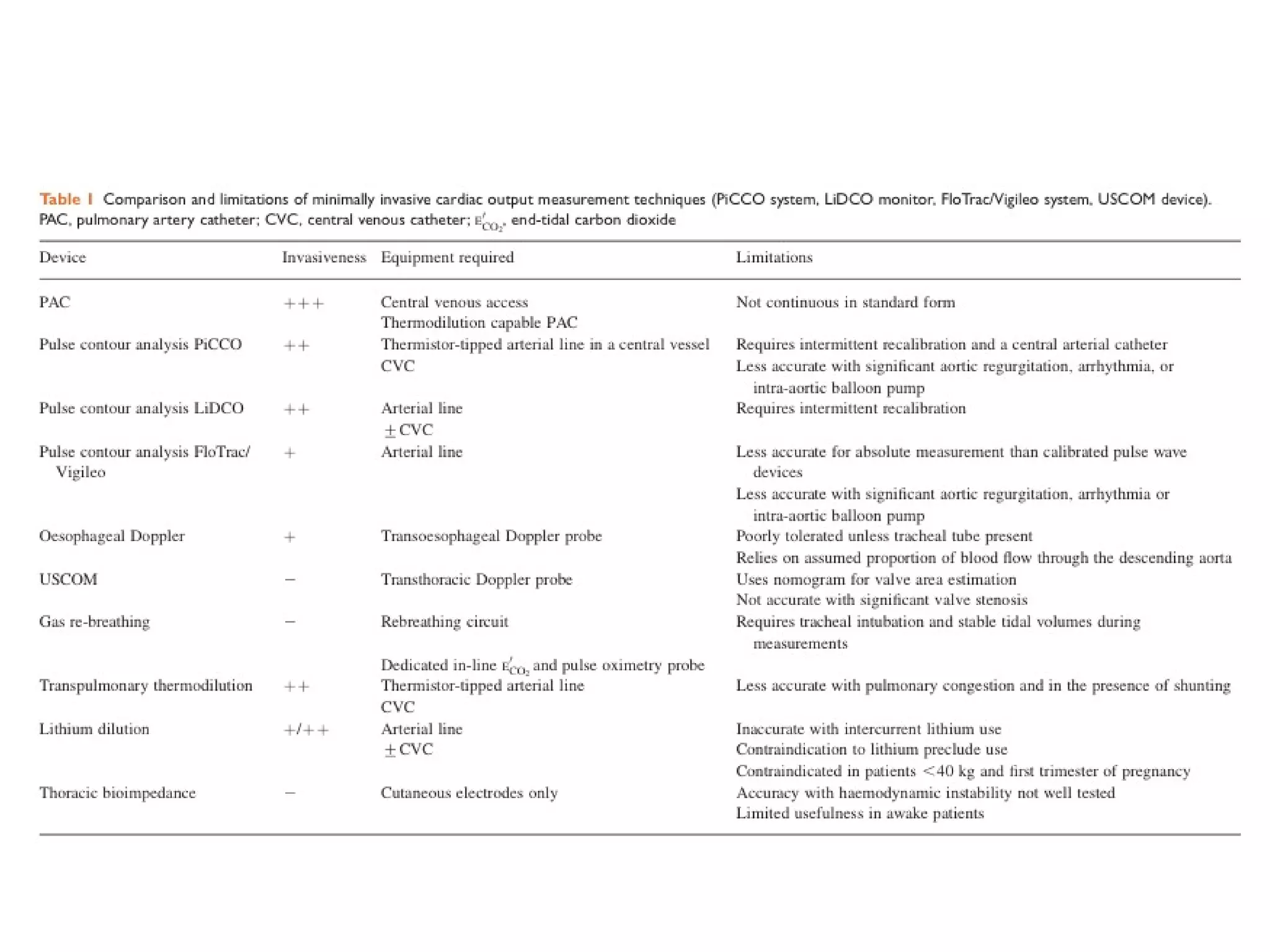
This document summarizes different methods for measuring cardiac output, including clinical assessment, minimally invasive techniques, and invasive pulmonary artery catheterization. Clinical assessment involves evaluating end organ perfusion rather than direct cardiac output measurements. Minimally invasive techniques discussed include thoracic bioimpedance and esophageal Doppler. Invasive pulmonary artery catheterization provides direct cardiac output measurements via thermodilution but carries risks of complications. The document evaluates the advantages, limitations, and evidence for various cardiac output monitoring methods.