Science PPT on Acids and Bases
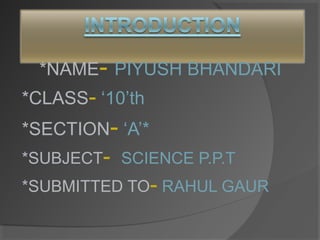
- 1. *NAME- PIYUSH BHANDARI *CLASS- ‘10’th *SECTION- ‘A’* *SUBJECT- SCIENCE P.P.T *SUBMITTED TO- RAHUL GAUR
- 2. Chapter -2
- 3. Acids An acid is a substance that produces hydrogen ions (H+ ) when placed in water When acids/ ionic compounds are dissolved in water, the ions split apart from each other (dissociation) Photo courtesy of: http://www.biologycorner.com/worksheets/acids_bases_coloring.html
- 4. Properties of Acids Most acids… Taste sour React w/many metals to form H2 gas Are corrosive (appears to “eat away” materials while reacting) Have a chemical formula that begins with H Photo Courtesy of: http://healthmad.com/conditions-and-diseases/the-surprising-health-benefits-of-lemons/ Zinc Metal + Hydrochloric Acid ?? Zn (s) + 2 HCl (aq) Predict the type of reaction based on the reactants! Single-Displacement H2 (g) + ZnCl2 (aq)
- 5. Common Acids • Citric Acid Found in citrus fruits • Used as a preservative • Lactic Acid Found in yogurt Produced by our muscles when they are overworked • Acetic Acid (HC2H3O2) Vinegar Used as a preservative • Carbonic Acid ( H2CO3) or Phosphoric Acid (H3PO4) Found in soft drinks • Hydrochloric Acid (HCl) Found in our stomachs- aids in food digestion Photo Courtesy of: http://stayinhealth.wordpress.com/2010/02/08/sugary-soft-drinks-increase-pancreas-cancer-risk
- 6. Bases A base is a substance that produces hydroxide ions (OH- ) when placed in water ○ When bases/ ionic compounds are dissolved in water, the ions split apart from each other (dissociation) Photo courtesy of: http://www.biologycorner.com/worksheets/acids_bases_coloring.html
- 7. Properties of Bases Most bases… Taste bitter Feel slippery Are corrosive (appears to “eat away” materials while reacting) Have a chemical formula that ends with OH Photo Courtesy Of: http://www.pbs.org/wgbh/nova/body/bitter-taste.html
- 8. Common Bases o Ammonia • Cleaners and fertilizers Sodium hydroxide • Used in soaps and drain cleaners Magnesium hydroxide • An ingredient found in antacids
- 9. Indicators Indicators are used to determine if a substance is an acid or a base An indicator changes a specific color when in the presence of an acid or a base Litmus Paper: indicator made of a special filter paper that contains dyes extracted from lichens (the crusty “stuff” that grows in rocks) Blue litmus turns red in an ACID Red litmus turns blue in a BASE Why do you think it’s important to test an unknown substance with both types of litmus paper? If one type of litmus paper does not change color, that does not guarantee that the other type of litmus paper will change color (i.e. water)
- 10. pH The pH scale can also help us classify solutions as acids or bases pH is a measure of the amount of H+ ions in a solution (potential hydrogen) The more H+ ions, the lower the pH, the more acidic the solution
- 11. pH Scale 7 < pH ≤ 14
- 12. Complete the following chart by telling whether the pH represents an acid, base or neutral substance. Also tell what color each type of litmus paper will turn at that pH level. pH Acid/Base/Neutral Color of Blue Litmus Paper Color of Red Litmus Paper 2 8 4 7 13
- 13. Strength vs Concentration When describing acids and bases, strength and concentration do not mean the same thing! Strength refers to the ability of the acid/base to dissociate in solution ○ pH measures this • Dissociation refers to the ability of an ionic compound to break apart into ions Concentration refers to the amount of acid/base dissolved in solution. ○ An acid or base will have the same pH, regardless of how concentrated it is • Just because you add water to dilute it, it’s still going to have the same amount of H+ ions, they’ll just be spread out more
- 14. Strong/Weak Acids & Bases When a strong acid/base dissolves in water, nearly all of the acid/base molecules will dissociate into ions The greater the ability to dissociate, the more potential the acid or base has for being dangerous because there are more ions available to react When a weak acid/base dissolves in water, only a small fraction of the acid/base molecules dissociate (dissociate partially) With less ions in solution, there is less potential for danger because there are less ions available to react Hydrogen Hydrogen
- 15. Strong/Weak Acids Examples Strong Acids 1. HCl – hydrochloric acid – stomach acid 2. H2SO4 – sulfuric acid – battery acid 3. HNO3 – nitric acid Weak Acids: 1. HC2H3O2 – acetic acid – vinegar 2. H3C6H5O7 –citric acid –citrus fruits
- 16. Strong/Weak Bases Examples Strong Bases: 1. NaOH – sodium hydroxide 2. Any alkali or alkaline earth metal with OH- (i.e. KOH – potassium hydroxide) Weak Bases: 1. Al(OH)3 – aluminum hydroxide – often found in deodorants 2. NH4OH – ammonium hydroxide (ammonia) –used in many cleaning products
- 17. Neutralization Neutralization is a chemical reaction between an acid and a base that takes place in a water solution When acids and bases react, the H+ ions from the acid react with the OH- ions from the base to form HOH (H2O - water!) The overall pH becomes “neutral” Many people that suffer from heartburn will take antacids to ease their symptoms (i.e TUMS). How do you think antacids work? The stomach is acidic due to HCl and antacids are basic. A neutralization reaction occurs!
- 18. Salts The ions that are left behind after the H+ and OH- ions form water, combine to form a salt A salt is a compound that forms when the negative ions from the acid combine with the positive ions from the base Acid + Base Water + Salt HCl(aq) + KOH(aq) H2O(l) + KCl(aq) What type of reaction is a neutralization reaction? Double-Displacement Name the salt produced in this neutralization reaction. Potassium chloride