requiredaction50202009211841519081.pdf
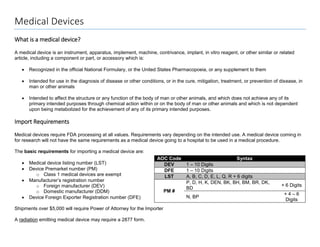
- 1. Medical Devices What is a medical device? A medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component or part, or accessory which is: Recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them Intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals Intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. Import Requirements Medical devices require FDA processing at all values. Requirements vary depending on the intended use. A medical device coming in for research will not have the same requirements as a medical device going to a hospital to be used in a medical procedure. The basic requirements for importing a medical device are: Medical device listing number (LST) Device Premarket number (PM) o Class 1 medical devices are exempt Manufacturer’s registration number o Foreign manufacturer (DEV) o Domestic manufacturer (DDM) Device Foreign Exporter Registration number (DFE) Shipments over $5,000 will require Power of Attorney for the Importer A radiation emitting medical device may require a 2877 form. AOC Code Syntax DEV 1 – 10 Digits DFE 1 – 10 Digits LST A, B, C, D, E, L, Q, R + 6 digits PM # P, D, H, K, DEN, BK, BH, BM, BR, DK, BD + 6 Digits N, BP + 4 – 6 Digits
- 2. Use the table provided on the next page to determine what is required for entry based on the intended use. 510(k) Premarket Notification: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm Establishment Registration & Device Listing: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfrl/rl.cfm Radiation Emitting Products: https://www.fda.gov/Radiation- EmittingProducts/ElectronicProductRadiationControlProgram/GettingaProducttoMarket/RegulatedProducts/default.htm Intended Use Description Requirements Medical use as a medical device DEV | DFE | LST Domestic Refurbishing DEV | DFE | LST Domestically manufactured device that is part of a medical device convenience kit DDM | DFE | LST Foreign manufactured device that is part of a medical device convenience kit DEV | DFE | LST Constituent part for use in a medical product regulated under a drug (CBER) application type DEV | DFE | LST Import of a medical device under enforcement discretion provisions per final guidance For personal use (shipper and receiver must both be individuals) For public exhibition, trade show, or display as a non-food product For charitable organization use DEV | DFE | LST Component for further manufacturing into a finished medical device Device component for use in a medical product regulated under a drug (CBER) application type For repair and re-exportation For research and development - research and development of a medical device For research and development - bench testing of nonclinical research use; Import of a Device Sample for Customer Evaluation For research and development - clinical investigational use IDE Import of a device that is US goods returned for refund/overstock (to the manufacturer) DDM | LST US goods returned for sale to a third party DFE | DDM | LST Compassionate Use/Emergency Use Device Import of a single-use device for domestic reprocessing DDM | LST Import of a multi-use device for domestic reprocessing Import for Export DEV | DFE | LST Import for Export of a medical device component DEV | DFE | LST Other DEV | DFE | LST
- 3. Please provide the Device Information in the chart(s) below for all devices contained in the shipment along with the devices the information applies to: SONOSITE MICROMAXX ULTRASOUND WITH CONVEX PROBE SONOSITE,INC. 21919 30TH DRIVE SE Bothell, WA 98021 -3904 1 1,850 $ K053069 3032367 U.S.A.
- 4. Sunglasses/Spectacles What is a medical device? A medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component or part, or accessory which is: Recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them Intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals Intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. Import Requirements Spectacles, sunglasses, and optical frames are medical devices and require FDA processing at all values. Requirements vary depending on the intended use. Sunglasses coming in for research will not have the same requirements as a sunglasses being imported for resale. The lens for eyeglasses and/or sunglasses must be certified as impact resistance under 21 CFR Part 801.410. The basic requirements for importing a sunglasses or eyeglasses are:
- 5. Medical device listing number (LST) Manufacturer’s registration number o Foreign manufacturer (DEV) o Domestic manufacturer (DDM) Device Foreign Exporter Registration number (DFE) Impact Resistance Certification (IRC) Shipments over $5,000 will require Power of Attorney for the Importer. Use the table provided on the next page to determine what is required for entry based on the intended use. AOC Code Syntax DEV 1 – 10 Digits DFE 1 – 10 Digits LST A, B, C, D, E, L, Q, R + 6 digits
- 6. Establishment Registration & Device Listing: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfrl/rl.cfm Sunglasses, Spectacle Frames, Spectacle Lens and Magnifying Spectacles: https://www.fda.gov/MedicalDevices/ucm150001.htm Certification Statement of Impact Resistance: https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm150002.htm Intended Use Description Requirements Medical use as a medical device DEV | DFE | LST Domestic Refurbishing DEV | DFE | LST Domestically manufactured device that is part of a medical device convenience kit DDM | DFE | LST Foreign manufactured device that is part of a medical device convenience kit DEV | DFE | LST Constituent part for use in a medical product regulated under a drug (CBER) application type DEV | DFE | LST Import of a medical device under enforcement discretion provisions per final guidance For personal use (shipper and receiver must both be individuals) For public exhibition, trade show, or display as a non-food product For charitable organization use DEV | DFE | LST Component for further manufacturing into a finished medical device Device component for use in a medical product regulated under a drug (CBER) application type For repair and re-exportation For research and development - research and development of a medical device For research and development - bench testing of nonclinical research use; Import of a Device Sample for Customer Evaluation For research and development - clinical investigational use IDE Import of a device that is US goods returned for refund/overstock (to the manufacturer) DDM | LST US goods returned for sale to a third party DFE | DDM | LST Compassionate Use/Emergency Use Device Import of a single-use device for domestic reprocessing DDM | LST Import of a multi-use device for domestic reprocessing Import for Export DEV | DFE | LST Import for Export of a medical device component DEV | DFE | LST Other DEV | DFE | LST
- 7. Please provide the Device Information in the chart(s) below for all devices contained in the shipment along with the devices the information applies to. Also please provide the Impact Resistance Certification Documents (Drop Ball) for Spectacles and Sunglasses contained in the shipment:
- 8. Contact Lenses Contact lens sales are regulated by both the FDA and the Federal Trade Commission (FTC). Before you buy any contact lenses from someone other than your eye care professional, the FDA wants you to be a wise consumer. With a valid contact lens prescription, it is possible to purchase your contact lenses from stores, the Internet, over the phone or by mail. Decorative contact lenses are medical devices. The U.S. Food and Drug Administration oversees their safety and effectiveness, just like contact lenses that correct your vision Import Requirements Contact Lenses require FDA processing at all values because they are medical devices. The requirements for importing contact lenses are: Medical device listing number (LST) Device Premarket number (PM) Manufacturer’s registration number o Foreign manufacturer (DEV) o Domestic manufacturer (DDM) Device Foreign Exporter Registration number (DFE) Copy of prescription Prescription must be valid at the time of entry. Expired prescriptions can’t be used for entry. ‘Colored’ and Decorative Contact Lenses: A Prescription is A Must: https://www.fda.gov/forconsumers/consumerupdates/ucm275069.htm Contact Lenses: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/ContactLenses/de fault.htm AOC Code Syntax DEV 1 – 10 Digits DFE 1 – 10 Digits LST A, B, C, D, E, L, Q, R + 6 digits PM # P, D, H, K, DEN, BK, BH, BM, BR, DK, BD + 6 Digits N, BP + 4 – 6 Digits
- 9. Contact Lens Prescription: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/ContactLenses/uc m062345.htm Please provide the Device Information in the chart(s) below for all devices contained in the shipment along with the devices the information applies to. Please provide a copy of the patient’s prescription of shipments of contacts travelling from a business to an individual: