Seminal Fluid Analysis
- 1. 1 Volume 1 Clinical Pathology Atlas 11.01. 2020 The study of microscopic view of clinical Pathology. Semen Analysis
- 2. [Title] 1 1 Clinical Pathological examination Clinical Pathological examination Technologist : Ajith.A DMLT Kanthalloor | Marayoor idukki kerala 8637499285 | ajitharun937@gmail.com
- 3. [Title] Seminal Fluid analysis The examination of the seminal fluid a study to determine the fertility of the man. A male patient has undergone in order to check on the completeness of the surgical infertility. Seminal fluid produced by the seminal vesicles the prostate gland and the cowpers glands and it serves as the vehicle to carry the Spermatozoa which are formed in the seminiferous tubules of the testis. The semen collected by the patient as it is ejaculated into a clear. Dry bottle and it should be brought to the lab as soon as possible. LEARNING OBJECTIVES After studying this chapter, the student should be able to: 1. Discuss the composition of seminal fluid and briefly describe the function of each of the following structures in seminal fluid formation: Epididymis Interstitial cells of Leydig Prostategland Seminalvesicles Seminiferoustubules 2. Outline the maturation of sperm (spermatozoa) and identify the morphologic structures in which each maturation phaseoccurs. 3. Summarize the collection of seminal fluid for analysis, including the importance of timing and recovery of the complete specimen. 4. Describe the performance of the physical examination (appearance, volume, and viscosity) of seminal fluid and the results expected from a normal specimen.
- 4. [Title] 5. Describe the procedures used to evaluate the following characteristics of sperm in seminal fluid, state the normal range for each parameter, and relate each function to male fertility: Agglutination Concentration Morphology Motility Viability 6. Identify and describe the morphologic appearance of normal and abnormal forms of spermatozoa. 7. Discuss the origin and clinical significance of cells other than sperm in the seminal fluid. 8. Discuss briefly the role of quantifying the following biochemical substances in seminal fluid and identify the structure evaluated by each substance: Acid phosphatase Citric acid Fructose pH Zinc Physiology Specimen Collection Physical Examination Appearance Volume Viscosity Microscopic Examination Motility Concentration and Sperm Count Post vasectomy Sperm Counts Morphology Vitality Cells Other Than Spermatozoa Agglutination Chemical Examination
- 5. [Title] pH and Fructose Other Biochemical Markers KEY T E R MS Epididymis A long, coiled, tubular structure attached to the upper surface of each testis that is continuous with the vas deferens. The epididymis is the site of final sperm maturation and the development of motility. Sperm are concentrated and stored here until ejaculation. interstitial cells of Leydig Cells located in the interstitial space between the seminiferous tubules of the testes. These cells produce and secrete the hormone testosterone. Lquefaction The physical conversion of seminal fluid from a coagulum to a liquid following ejaculation. Prostate gland A lobular gland surrounding the male urethra immediately after it exits the bladder. The prostate is an accessory gland of the male reproductive system and is testosterone dependent. It produces a mildly acidic secretion rich in citric acid, enzymes, proteins, and zinc that is added to ejaculates. seminal fluid (also called semen) A complex body fluid that transports sperm. Seminal fluid is composed of secretions from the testes, epididymis, seminal vesicles, and prostate gland. seminal vesicles Paired glands that secrete a slightly alkaline fluid, rich in fructose, into the ejaculatory duct. Most of the fluid in the ejaculate originates in the seminal vesicles.
- 6. [Title] seminiferous tubules Numerous coiled tubules located in the testes. The seminiferous tubules are collectively the site of spermatogenesis. Immature and immotile sperm are released into the seminiferous tubular lumen and are carried by its secretions to the epididymis for maturation. sperm (also called spermatozoa) Male reproductive cells. vasectomy A procedure in which both vas deferens are surgically severed and at least one end of each is sealed. It is a male sterilization procedure because it prevents sperm from becoming part of the ejaculate. viscosity A measure of fluid flow or its resistance to flow. Low- viscosity fluids (e.g., water) flow freely and form discrete droplets when expelled drop by drop from a pipette. In contrast, high- viscosity fluids (e.g., corn syrup) flow less freely and do not form discrete droplets; rather they momentarily form threads or strings when expelled from a pipette. Seminal fluid or semen is a complex body fluid used to transport sperm or spermatozoa. It is analyzed routinely to evaluate infertility and to follow up after a vasectomy to ensure its effectiveness. Other reasons for analysis include the evaluation of semen quality for donation purposes and forensic applications (e.g., DNA analysis, detection of semen). Familiarity with the male reproductive tract and its various functions facilitates understanding of the physical, microscopic, and biochemical abnormalities that can occur in semen .
- 7. [Title] PHYSIOLOGY Semen is composed primarily of secretions from the testes, epididymis, seminal vesicles, and prostate gland, with a small amount derived from the bulbourethral glands. The biochemical composition of semen is complex. Although the specific functions of some components (e.g., fructose) are known, the functions of others (e.g., prostaglandins) remain uncertain. The testes are paired glands suspended in the scrotum and located out side the body(Figure11-1). Their external location
- 8. [Title] allows for the lower organ temperature necessary for sperm formation. The testes perform both an exocrine function by the secretion of sperm and an endocrine function by the secretion of testosterone. These two functions are interdependent and are regulated by two pituitary hormones: follicle-stimulating hormone and luteinizing hormone. The cells responsible for these two functions are distinctly different. Sperm production is regulated by Sertolicells in the seminiferous tubules, where as production and secretion of the male sex hormone, testosterone, is the responsibility of the interstitial cells of Leydig, which are located in the interstitium of the testes,between the seminiferous tubules.
- 9. [Title] Sertoli cells of the seminiferous tubular epithelium have several functions. Because of their tight interconnections, they essentially form a barrier that separates the epithelium into two distinct compartments: the basal compartment (i.e., germ cell layer) and the ad luminal compartment(i.e., epithelium near at the tubular lumen). As this barrier or gatekeeper, they limit the movement of chemical substances from the blood into the tubular lumen—playing a role in supplying nutrients, hormones, and other substances necessary for normal spermatogenesis. They also control the movement of spermatocytes from the germ cell layer into the ad luminal compartment, and last, they continuously produce a fluid that carries the newly produced immotile sperm into the lumen of the seminiferous tubules and on to the epididymis. The epithelium of the numerous coiled seminiferous tubules consists of Sertoli and germ cells. The un- differentiated germ cells (spermatogonia) continuously undergo mitotic division to produce more germ cells. At the same time, some of them move slowly toward the tubular lumen, changing in size and undergoing meiotic (reduction) division until they form spermatids. Figure11- 2depictsspermatogenesisintheseminiferoustubular epithelium with all stages of spermatogenesis depicted. Spermatogonia (germ cells) evolve into spermatocytes, then spermatids. With nuclear modification and cellular restructuring, spermatids ultimately differentiate into immotilesperm. When Sertoli cells release sperm into the lumen of the seminiferous tubules, they are non motile and still immature. Luminal fluid from Sertoli cells carries the sperm into the tubular network of the epididymis, where they undergo final maturation and become motile. The epididymis also adds carnitine and acetylcarnitine to the lumen fluid. Although the exact function of these chemi- cals remains to be elucidated, abnormal levels of them have been associated with infertility. Other functions of the epididymis include the concentration of sperm by the absorption of lumen fluid and their storage until ejaculation. Following a vasectomy, the epididymisis the site of leukocyte infiltration and phagocytization of accumulated sperm.
- 10. [Title] The epididymis ultimately forms a single duct that joins the vas deferens. The vas deferens is a thick-walled muscular tube that transports sperm from the epididymis to the ejaculatory duct, and the dilated end of the vas deferens is located inferior to the bladder. Secretions from the seminal vesicles are added at the ejaculatory duct. Both ejaculatory ducts then pass through the prostate gland and empty into the prostatic urethra along with secretions from the prostate. All structures preceding the prostate gland are paired (e.g., two ejaculatory ducts, two seminal vesicles, two testes). The seminal vesicles and the prostate gland are considered accessory glands of the male reproductive system and are testosterone dependent. They produce and store fluids that provide the principal transport medium for sperm. Seminal vesicle fluid accounts for approximately 70% of the ejaculate and is high in flavin. Flavin imparts the characteristic gray or opalescent appearance to semen and is responsible for its green- white fluorescence under ultraviolet light.1Another characteristic of seminal vesicle fluid is its high concentration of fructose, believed to serve as a nutrient for spermato- zoa. The various proteins secreted by the seminal vesicles play a role in coagulation of the ejaculate, whereas the function of prostaglandins remains under investigation. (Prostaglandins were originally thought to be a prostatic gland secretion, hence their misnaming.) Prostatic fluid secretions account for approximately 25% of the ejaculate volume. The principal components of this milky, slightly acidic fluid are citric acid; enzymes, particularly acid phosphatase and proteolytic enzymes; proteins; and zinc. Semen is unique in its high concentration of the enzyme acid phosphatase. Hence acid phosphatase activity can be used to positively identify the presence of this body fluid. Proteins and some enzymes in prostatic secretions play a role in coagulation of the ejaculate, whereas the proteolytic enzymes are responsible for its liquefaction. Zinc is primarily added to semen by the prostate gland; however, the testes and sperm also contribute zinc. Semen zinc levels can be used to evaluate prostate function; a decreased level is associated with prostate gland disorders.
- 11. [Title] In summary, semen is a highly complex transport medium for sperm. The paired seminal vesicles and the single prostate gland are the major fluid contributors to semen. Sperm produced by the testes are matured and concentrated in the epididymis, and make up only a small percentage of an ejaculate. Dilution of sperm by the relatively large volume of seminal fluid at ejaculation enhances sperm motility. Without adequate dilution, sperm motility is significantly reduced. The entire process of spermatogenesis and maturation (i.e., from primary spermatocyte to mature motile spermatozoon) takes approximately 90 days. SPECIMENCOLLECTION Because sperm concentration in normal seminal fluid can vary significantly, two or more samples should be analyzed to evaluate male fertility. Specimen collections should take place within a 3-month period and at least 7 days apart. Sexual abstinence for at least 2 days (48 hours), but not exceeding 7 days, should precede the collection. The patient collects the specimen through masturbation, and the entire ejaculate is collected in a clean, wide-mouth sterile plastic or glass container. Although some plastic containers are toxic to spermatozoa, others are not. Sterile urine specimen or similar containers are often satisfactory but the laboratory must evaluate them before their use.2The collection container should be kept at room temperature or warmed (to approximately body temperature) before the collection to avoid the possibility of cold shock to the sperm. The container can be warmed easily by holding it next to the patient’s body or under the arm for several minutes before the collection. This technique can also be used to control the temperature of specimens being transported in cold climates. Specimen containers and request forms must be labeled with the patient’s name, the period of sexual abstinence, and the date and time of specimen collection. The time of actual specimen collection is crucialin evaluating liquefaction and sperm motility. During specimen collection, lubricants and ordinary condoms should not be used because they have spermicidal properties. For patients unable to collect a specimen through masturbation, special non spermicidal (e.g., Silas- tic)condoms can be provided for specimen collection.
- 12. [Title] The collection of seminal fluid requires sensitivity and professionalism. Written and verbal instructions should be provided to the patient, as well as a comfortable and private room near the laboratory. If the specimen is to be collected elsewhere and delivered to the laboratory, clearly written in structions regarding specimen transport conditions must be provided. Specimens must be received in the laboratory within 1 hour following the collection, and they must be protected from extreme temperatures, that is, maintained between 20° C and 40° C.3If these criteria are not met, the specimen will not be satisfactory for sperm function tests and an abnormally low sperm motility can result. Because the ejaculate differs in its composition, only complete collections are acceptable for analysis. Patient instructions must state this clearly, and patients should be asked whether any portion of the specimen was not collected. When a portion of the initial ejaculate is not collected, the sperm concentration will be falsely decreased, and owing to are duct ion in prostate secretions, the pH is falsely increased and the coagulum will fail to liquefy. Conversely, when the last portion of an ejaculate is missing (primarily seminal vesicle fluid), the semen volume will be decreased, the sperm concentration falsely increased, and the pH falsely decreased, and a coagulum will not form.
- 13. [Title] As with all body fluids, seminal fluid represents a potential biohazard and must be handled accordingly. Because seminal fluid can contain infectious agents such as hepatitis virus, human immunodeficiency virus, herpes virus, and others, all personnel mustad here to Standard Precautions (see Chapter 2) when handling these specimens. PHYSICALEXAMINATION Appearance Normal semen is gray-white and opalescent in appearance. A brown or red hue may indicate the presence of blood, whereas yellow coloration has been associated with
- 14. [Title] certain drugs. If large numbers of leukocytes are present, the semen appears more turbid with less translucence. When the specimen appears almost clear, the sperm concentration is usually low. Mucus clumps or strands can be present. Semen has a distinctive odor that is some times described as musty. Although infections in the male reproductive tract can modify this odor, a change is rarely noted or reported. Table 11-1 (and Appendix B) summarizes the semen (physical, microscopic, and chemical parameters) associated with fertility. Semen is a homogeneous viscous fluid that immediately coagulates after ejaculation to form a coagulum. Within 30 minutes, the coagulum liquefies (becomes watery). The actual time of specimen collection must be known to evaluate liquefaction. Although liquefaction can take longer, any delay beyond 60 minutes is considered abnormal and must be noted. Because complete liquefaction is necessary to perform analysis, semen specimens that do not liquefy completely can be chemically treated (see Appendix C, Semen Pretreatment Solution). Following normal liquefaction, undissolved gel–like granules or particles can be present in the specimen, with a small amount considered normal. Volume The physical and microscopic analyses of seminal fluid should take place immediately following liquefaction or within 1 hour after collection (for specimens collected away from the laboratory). Specimen volume is mea sured to one decimal place (0.1 mL) using a sterile serologic pipette (5.0 mL or 10.0 mL). If a semen culture for bacteria is requested, the volume measurement should be performed first using sterile technique. Normally, a complete ejaculate collection recovers 2 to 5 mL of seminal fluid. Volumes less than and greater than this range are considered abnormal and have been associated with infertility.
- 15. [Title] Viscosity After complete liquefaction, the viscosity of the semen is evaluated using a Pasteur pipette and observing the droplets that form when the fluid is allowed to fall by gravity. A normal specimen is watery and forms into discrete droplets. Abnormal viscosity or fluid thickness is indicated by the formation of a string or thread greater than 2 cm in length.3A high mucus content can increase the viscosity. Other conditions associated with increased viscosity include the production of anti sperm antibodies and oligoasthenospermia (i.e., decreased concentration and motility of sperm).4-7 Grading viscosity varies among laboratories. Numeric terms can be used, with 0 indicating a normal, watery (i.e., forms discrete drops) specimen, and 4 indicating a specimen with gel-like consistency.8An alternate reporting format uses descriptive terms, such as normal, slightly viscous(thick),moderately viscous, and extremely viscous (unable to be as pirated into the pipette). CHEMICAL EXAMINATION pH The pH of fresh normal semen is alkaline and ranges from 7.2 to 7.8. Fresh specimens with a pH less than 7.2can be obtained from individuals with abnormalities of the epididymis, the vas deferens, or the seminal vesicles. In contrast, fresh specimens exceeding pH 7.8 suggest an infection in the male reproductive tract. Specimens not tested within 1 hour of collection can show changes in the pH for several reasons. An increase in pH can occur because of loss of carbon dioxide; conversely, a decrease in pH can occur because of the accumulation of lactic acid, particularly in specimens with a high sperm count.2Despite the limited usefulness of a seminal fluid pH, the
- 16. [Title] measurement is easy to determine and is usually included in a seminal fluid analysis. Commercial pH paper strips with a range from 4.0 to 10.0 should be used and results recorded to the nearest 0.1 pH unit. Appropriate quality control solutions should be used to ensure the accuracy of the pH strips. Fructose The determination of fructose in semen is a commonly performed chemical test. Because fructose is produced and secreted by the seminal vesicles, its presence in semen reflects the secretory function of this gland and the functional integrity of the ejaculatory ducts and vasdeferens. The fructose level is most often determined when the sperm count reveals azoospermia (i.e., no sperm). Obstruction of the ejaculatory ducts or abnormalities of the seminal vesicles or vas deferens can cause low fructose levels and azoospermia.Normally, semen fructose levels are equal to or greater than 13 µmol per ejaculate. Several quantitative, spectrophotometric procedures are available for fructose determinations. A rapid and easy qualitative tube test based on the development of an orange-red color in the presence of fructose can also be performed.2With this test, failure of the specimen to develop an orange-red color indicates the absence of fructose. Although this technique is qualitative, relies on the visual assessment of color, and lacks sensitivity to decreased fructose levels, its ease of performance and rapid turnaround time make it a useful tool. Other Biochemical Markers Quantitative determinations of zinc and citric acid levels in semen can be used to evaluate the secretory function of the prostate gland. The usefulness of zinc and citric acid measurements as markers of biochemical function is ongoing; clinicians are attempting to establish correlations with disease processes (e.g., low zinc levels with
- 17. [Title] prostatitis). Quantitation of zinc can be per-formed by spectrophotometric or atomic absorption spectroscopy techniques. In normal semen, the total zinc concentration is equal to or greater than 2.4 mmol per ejaculate. Citric acid, the major anion in semen, can be quantitated using spectrophotometric methods.1Decreased levels indicate dysfunction of the prostate gland. The total citric acid concentration in normal semen is equal toorgreaterthan52mmolperejaculate. Acid phosphatase activity is a useful marker to assess the secretory function of the prostate gland. Normally, seminal fluid contains 200 units of enzyme activity or more per ejaculate, whereas other body fluids contain insignificant amounts. Because of this uniquely high con- centration, prostatic acid phosphatase measurements are often used to determine whether semen is present in vaginal fluid specimens obtained from women following an alleged rape or sexual assault. Even washings of the skin or stained clothing can reveal significant levels of prostatic acid phosphatase, which positively identifies the presence of semen. Otherbiochemicalsubstancesarebeinginvestigatedinanattempttoidentifyandestablishs pecificmarkersformalereproductivetractabnormalities.Forexample,l-carnitineandα- glucosidasearebeingevaluatedasindicatorsofepididymalfunction,whereasspecificlact atedehydrogenaseisoenzymesofspermarebeingexaminedfortheirclinicaluseintheeval uationofmalefertility. MICROSCOPIC EXAMINATION As in other laboratory areas, the standardization of procedure sand techniques is necessary to enhance the precision and reproducibility of semen analysis. Once achieved, this standardization enables intralaboratory and inter- laboratory comparisons of data. Appropriate quality control measures must also be in place whenever applicable. The World Health Organization publication WHO Laboratory
- 18. [Title] Manual for the Examination and Processing of Human Semen is an excellent reference for any laboratory performing semen analysis.3Microscopic examination includes the determination of sperm motility, concentration, morphology, and viability; the concentration of other cells present; and the presence of sperm agglutination. Some laboratories use a single stain for the evaluation of several parameters, such as eosin nigrosin stain for sperm vitality, morphology, and the identification of other cells, whereas others use different stains that specifically enhance each parameter to aid in the identification and evaluation of sperm and other cells. Motility Motility is one of the most important characteristics of sperm because immotile sperm, even in high concentrations, are unable to reach and fertilize an ovum. Tradition-ally, the evaluation of sperm motility has been assessed subjectively by experienced technologists. Today, computerized systems that use electro-optical techniques or videography have been developed for semen evaluation. This advanced technology enables objective evaluation of sperm motility and morphology; however, the cost of the equipment precludes many laboratories from acquiring it. Without an automated system, sperm motility is evaluated subjectively and semi quantitatively using phase-contrast microscopy (brightfield microscopy can also be used with appropriate condenser adjustments). After complete liquefaction, the semen sample is mixed well to ensure homogeneity. A consistent volume of each specimen is evaluated by pipetting a fixed volume (e.g.,10 or 20 µL) of semen onto a microscope slide using a calibrated positive-displacement pipette. The sample is covered with a coverslip of predetermined size (e.g., 18× 18 mm), and the slide is allowed it to settle for about 1 minute before evaluation. To enhance the accuracy and precision of results, wet mounts of each sample should be prepared and evaluated in duplicate. Because sperm motility is affected adversely by temperature, some laboratories control the temperature of the microscope slide at 37° C using an air curtain Incubator.8Others.8Others.8Others.8Others perform the analysis at room
- 19. [Title] temperature (i.e., 22 ± 2° C). Initially, each wet mount is screened to ensure uniformity in sperm movement throughout the preparation. Next, sperm motility is graded subjectively from 0 to 4 under 200× (or 400×) magnification. Table 11- 2shows typical grading criteria used to evaluate sperm motility. Some laboratories use a manual cell counter and evaluate the motility characteristics in 100 sperm, whereas others grade the sperm encountered in 6to10 high-power fields (400×). The speed and forward progression of each sperm are evaluated. In normal semen evaluated within 60 minutes of collection, 50% or more of the sperm will show moderate to strong linear or forward progression. The prac- tice in some laboratories of reassessing sperm motility at additional time intervals serves no purpose and has no clinical significance. Physiologically or in vivo, sperm leave the seminal fluid within minutes following ejacula- tion and enter the cervical mucus. Therefore, motility on a microscope slide at later time intervals is irrelevant. Concentration and Sperm Count For fertility purposes, the actual number of sperm is not as important as other characteristics. This fact is sup- ported by studies offer tile men despite low spermcounts (fewer than 1 million per milliliter). The concentration of sperm in an ejaculate is considered normal when 20 to 250 million per milliliter of sperm are present; values less than or greater than this range are considered abnormal and are associated with infertility. The variation in the sperm concentration within a single individual can be significant and depends partially on the period of sexual abstinence but can also be affected by viral infection and stress. For these reasons, multiple semen specimens should be evaluated to reliably assess the quantity and quality of an individual’s sperm. Manually, the concentration of sperm is determined by using a hemacytometer after preparing an appropriate dilution of the semen. Frequently, a 1 : 20 dilution is prepared. If during initial microscopic examination, the sperm concentration is noted to be exceptionally high or low, a new dilution can be prepared and mounted. All dilutions should be made using a calibrated positive- displacement pipette to deliver the semen quantitatively to a premeasured amount of diluent (see Appendix C for diluents). Note that a hematology white blood cell pipette is not
- 20. [Title] accurate for use with seminal fluid because of its viscosity and should not be used.3After the hema- cytometer is filled with the well-mixed dilution of semen, it is placed in a humidifying chamber and allowed to settle for 3 to 5 minutes before counting. The type of hemacytometer, the specimen dilution used, and the areas counted determine the conversion factor necessary to obtain the concentration of sperm in millions per milliliter (seeChapter18forproceduraldetails). Several alternative manual counting methods have been developed, such as the Makler chamber (Mid- Atlantic Diagnostics, Mt Laurel, NJ), Horwell, Cell VU chambers (Millennium Sciences, NY), Microcell slides (Conception Technologies, San Diego, CA), and Leja slides (Leja, The Netherlands). Studies vary in their outcomes—some supporting the manual hemacytometer method as the method of choice for sperm counting, other studies found better accuracy and precision using an alternative counting chamber.4,5,10Regardless of the method used, the dilution of these men is always sapotential source for error and requires the utmost attention to ensure accurate and reproducible technique. The counting of motile sperm and high sperm concentrations have also been identified as two sources of error. Therefore the World Health Organization (WHO) states that the “validity of these alternative counting chambers must be established by checking chamber dimensions, comparing results with the improved Neubauer haemocytometer method, and obtaining satisfactory performance as shown by an external quality control program. 3In con- trast to sperm concentration (sperm per milliliter), the sperm count is the total number of sperm present in the entire ejaculate. This value, often requested by clinicians, is calculated by multiplying the sperm concentration (sperm/mL)by the total volume of the ejaculate. MICROSCOPICE SPERM COUNT
- 21. [Title] Procedure After liquefaction mix the semen sample thoroughtly and take 0.02 mL 20 µm (micro liter) in a test tube and dilute it with 0.38 mL of the diluting fluid. Gently mix and wait for 5 mts in room temperature. The diluting fluid is made as follows. Sodium bicarbonate - 5g Phenol or (formalin) - 1 mL Distilled water - 100 mL Procedure Charge the counting chamber (Improved, Neubauer ruling) and allows the sperm to settle to the surface of the chamber. And then count the number of sperm in the four corners squares. The formula for calculation of the sperm count is similar to the formula used in the WBC Count. We report the sperm count as the no. of sperm per milliliter (CC), instead of per micro liter (um mm) so that an additional multiplication faster of 1000 is added. Formula Sperm / mL = N × 20 × 10 × 1000 4 Normal value 60,000,000 – 150,000,000 /mL (60 – 150 million /mL ) Some authorities say the normal range is 100 million – 150 million / mL Others
- 22. [Title] The presence of other cells should also be noted these could included RBC's and pus cells and epithelial cells. Terminologies ASPERMIA : Absence of semen ( zero volume ) AZOOSPERMIA : Complete absence of spermatozoa OLIGOZOOSPERMIA : Count < 20 million / mL NECROZOOSPERMIA : All spermatozoa are dead from. CRYPTOZOOSPERMIA : Count < 1 million / 1 mL ASTHENOZOOSPERMIA : Motility < 40% Vitality Vital staining of a fresh semen smear enables rapid differentiation of live and dead sperm. Because dead sperm have damaged plasma membranes, these cells take up stain; living sperm do not (Figure 11-5). When a large percentage of immotile sperm are observed, this evaluation determines whether the sperm are immotile because they are dead or because of a structural abnormality (e.g., defective flagellum). Eosin alone or an eosin-nigrosin (a modification of Blom’s technique) combination is frequently used to determine sperm vitality. Using brightfield or phase- contrast microscopy and 1000× (or 400×), 100 sperm on a stained smear are evaluated. The percentage of dead sperm cells should not exceed the percentage of immotile sperm.
- 23. [Title] In other words, if 65% of the sperm in a semen specimen are dead, the motility cannot exceed 35%. Hence the vitality evaluation provides a convenient quality or cross- check of the motility evaluation. In fresh normalsemen,50% or more of the sperm are alive. Cells Other Than Spermatozoa An ejaculate is a complex mixture biochemically and cellularly. Ejaculates normally contain cells other than sperm, such as urethral epithelial cells, white blood cells (WBCs), and immature spermatogenic cells (i.e., spermatids, spermatocytes, and spermatogonia), as well as particulate matter and cellular debris. The spermatogenic cells can be difficult to differentiate from WBCs because of size and nuclear pattern similarities. A peroxidase stain can aid in this evaluation because neutrophils are peroxidase-positive cells, whereas lymphocytes and spermatogenic cells are peroxidase-negative cells. However, owing to the carcinogenicity of the chemicals used in many peroxidase stains and the special handling required, Wright’s stain may be preferred. The presence of greater than 1 million WBCs per milliliter of ejaculate indicates an inflammatory process, most often involving the male accessory glands (e.g., seminal vesicle, prostate). However, a normal WBC count does not rule out infection. Note that the concentrations of WBC and spermatogenic cells can be deter- mined after the spermcount using the same hemacytometer preparation (see Chapter 18). When the concentrations of these cells exceed 1 million per milliliter, a stained smear (e.g., Wright’s stain, peroxidase stain) of the fresh ejaculate is evaluated. Using this smear, the numbers of WBCs and immature spermatogenic cells are counted in the same fields used to count 100 mature sperm. With the sperm count (S) and by using the following equation, the concentration (C) of these cell types (N) is deter- mined (Equation11-2)3: Equation 11-2
- 24. [Title] C N S 100 Immature spermatogeniccells are present in the semen when they are exfoliated prematurely from the germinal epithelium of the seminiferous tubules. Distinguishing between an increase in WBCs and an increase in immature spermatogeniccells is necessary to evaluate infection and infertility. Red blood cells normally are not present in seminal fluid. If their presence is apparent during various aspects of the microscopic evaluation, it should be reported. Similarly, the finding of bacteria in semen should be reported. Bacteria do not normally reside in the male reproductive tract. However, collection of semen by masturbation makes bacterial contamination difficult to avoid. Agglutination Agglutination, the sticking together of motile sperm, is evident by microscopic examination of a wet preparation. Although some clumping of immotile sperm may occur in normal semen specimens, the observation of distinct head-to-head, head-to- tail, or tail-to-tail orientation of sperm is associated with the presence of sperm- agglutinating antibodies. Clumping of sperm with other entities, such as mucus and other cell types, is not identified as agglutination. The extent of true agglutination is often graded as “few,” “moderate,” or “many.” Even a small amount of true agglutination is significant and indicates the need for further evaluation. Immunoglobulin G and immunoglobulin A antibodies bound to sperm have been identified and correlated with reduced fertility. This is known as immunologic infertility; the man or the woman can produce anti sperm anti- bodies, and the source can be identified. When the man is producing them, the antibodies are present on the surface of the sperm before intercourse; when the woman is producing them, the sperm are coated with antibodies after they enter the cervical mucus.
- 25. [Title] Macroscopic and microscopic tests are available to detect and determine the immunoglobulin class of sperm antibodies ([Ig]G, IgA).3Both tests produce comparable results, but the mixed agglutination reaction (MAR) test is rapid (≈3 minutes) and easy to perform, whereas the immunobead test is time-consuming (≈45 minutes), technically more complicated, and more expensive. The cutoff values for these tests vary between laboratories. The WHO defines agglutination as clinically significant (abnormal) when anti sperm antibodies coat 50% or more of the spermatozoa, whereas other institutions use lower cutoffs(e.g.,20%,10%).13 Reporting format Color : Grayish White, Clear – Watery Volume : 3 ML Viscosity : Normal, Abnormal Liquefaction Time : Minutes Total sperm count : millions Rapid Motile : 70% Slagish : 25 % Non Motile : 05 % Pus cells : 2 - 3 RBC's : 0 - 1 Others : Fungus, Parasites any others Morphologycal Examination of Spermatozoa:
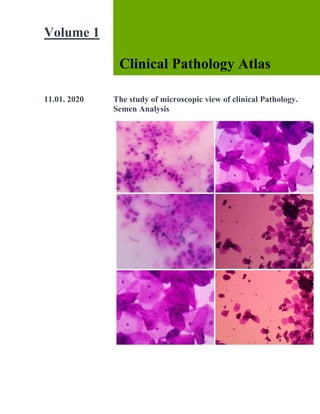
- 26. [Title] After 200 spermatozoa examined in oil immersion and the percentage of abnormal forms recorded in addition to sperm morphology the presence of ref blood cells, leucocytes and epithelial should be noted. The normal spermatozoa are 50 to 70 µm in length with large oval head and small neck or body and a long slender tail. Abnormaly shaped head Abnormaly sized head Double head Irregular distribution of the chromatin in the nucleus of the head Enlargement bifurcation or absence of the neck or middle section. Abnormalities of the tail, which may be very short, absent or double.
- 27. [Title] The normal 3 sperm cells, microsperms 2, a single irregular head and detached head 2. A single small acrosomal sperm cell, Two neck bent sperm, Two detached sperm and Two single RBC seen. The semen stain in simple stain the sperm cells admixed in the field.
- 28. [Title] Staining method The sperm can be stained with any simple stain even Leishmanstain. It can be stained with 0.25% aqueous basic fuchsin for 5 mts. Both gram stain and AFB stain can be done on fixed smears of the semen. Sperm morphology, like motility, is routinely assessed subjectively. Hence this qualitative determination is subject to intralaboratory and interlaboratory variations. To minimize these variations, standardized procedures and grading criteria must be established by each laboratory and adhered to by all laboratorians. Because the technical ability to identify and classify various morphologic forms requires experience, new staff members must be trained appropriately and their initial work reviewed to ensure accuracy and consistency in reporting. Sperm morphology is complicated by the wide variation in abnormal forms that can be encountered, and an inexperienced observer can easily miss subtle abnormalities in sperm. The computerized systems used to assess sperm motility can also evaluate sperm morphology. Sperm morphometry—measurement of the sperm head length, width, circumference, and area—enables the generation of objective data. To be considered normal, sperm must meet strict criteria regarding their size and shape, which can be determined by computerized systems or manually using a microscope with a calibrated ocularmicrometer. Human sperm have three distinct areas: head, mid- piece, and tail. When viewed from the side, sperm appear to be arrowhead shaped (Figure 11-3). When viewed from the top, normal human sperm have oval heads that are 2.5 to 3.5 µm in width and 4.0 to 5.0 µm in length. Only sperm lying flat should be evaluated and their head length-to- width ratio should be 1.50 to 1.75. Spermatozoa with values outside these ranges are
- 29. [Title] considered abnormal, and studies have shown statistically significant differences in the head length-to-width ratios of sperm from ejaculates of fertile and infertile men.6 The midpiece, located immediately behind the head, is 6 to 7.5 µm long and is thicker than the tail, but not greater than 1 µm in width. The tail should be slender, uncoiled, and at least 45 µm long. When a “basic” morphology evaluation is performed, each spermatozoon (single sperm cell) is identified simply as normal or abnormal with the percent of normal forms reported. If a “complete” morphology evaluation is performed, then each spermatozoon is classified using five categories: normal, head defects, mid piece defects, tail defects, and cytoplasmic droplet present. Cytoplasmic droplets are usually located in the mid piece region and are considered abnormal if this region is greater than one-third the area of a normal sperm head. The head can contain vacuoles, but they are not considered abnormal unless they occupy more than 20% of the head. Note that a single spermatozoon can have multiple defects, and each deficits. To manually evaluate sperm morphology, smears of fresh semen are made, air dried, and stained. The smears can be made similar to those for traditional blood smears by placing a drop (10 to 15 µL) of semen near one end of a clean microscope slide. Using the edge of another slide, the drop is allowed to spread along the edge of the second slide, and then the edge of the second slide is moved forward, dragging the semen sample across the surface of the first slide and producing a smear. An alternate technique involves placing the second slide over the first and allowing the semen to spread between them. Once spreading is complete, the slides are pulled apart and allowed to air dry. Staining enhances the visualization of sperm morphology and enables the identification and differentiation of white blood cells, epithelial cells of the urethra, and immature spermatogenic cells (i.e., spermatids, spermatocytes, and spermatogonia).Giemsa, Wright’s, and Papanicolaou stains are frequently used. These stains differ with respect to complexity and turn- around time, hence laboratories select the stain that best suits their needs and resources. Using oil immersion (1000×) and an area of the slide where sperm are evenly distributed, 200 sperm are classified. Note that morphologically abnormal sperm are
- 30. [Title] found in all semen specimens. Abnormalities may involve all or only one region of the spermatozoon and can affect its size, shape, or both. In addition, numerous sperm variations are found within a single ejaculate. Although some morphologic abnormalities have been associated with particular disorders (e.g., tapered heads with varicocele), most abnormalities are nonspecific. The reference range associated with normalcy varies with the criteria and the rigor used to evaluate sperm morphology. In some laboratories, a normal sperm morphology of 50% or greater is considered “normal.” However, when strict evaluation criteria are used for fertility purposes as in studies of fertile and sub fertile individuals, the number of sperm with normal morphology is significantly lower. In these studies, normal sperm morphology of less than 5% is a strong predictor of infertility, whereas fertility is associated with normal sperm morphology values of 12% to 15% or greater.12Between fertile and sub fertile individuals, wide overlap exists in the percentage of sperm with normal morphology. Other variables, particularly sperm concentration and progressive motility, combined with sperm morphology provide the greatest predictive value in assessing male fertility. Post vasectomy Sperm Counts Following a vasectomy, the sperm count in semen ideally should be zero—no sperm present (azoospermia)— within 12 weeks after the procedure. However, studies
- 31. [Title] have shown that non motile sperm can be present for as long as 21 months post vasectomy regardless of the number of ejaculations. It is postulated that the persistence or reappearance of non motile sperm in semen col- lections results from the release of nonviable residual sperm in the seminal vesicles and the abdominal portion of the vas deferens. Studies have further demonstrated that despite the presence of low numbers (<1 × 106) of non motile sperm post vasectomy, these individuals have a very low risk of causing pregnancy (i.e., comparable with azoospermic men).11 In clinical practice, most men (≈66%) demonstrate azoospermia within 12 weeks, regardless of the number of ejaculations. Note that the most important feature is not the number of sperm present post vasectomy but the status of their motility. The presence of even a single “motile” spermatozoon is evidence of an unsuccessful vasectomy (i.e., recanalization of the vas deferens has occurred), whereas low numbers of “immotile” sperm can persist for months in some men (≈33%).11