biopharceutics ppt (IHSBC).pptx
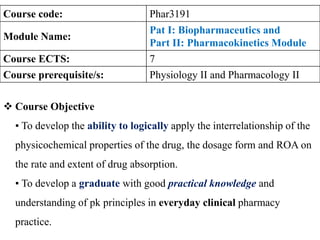
- 1. 12/14/2022 1 Course code: Phar3191 Module Name: Pat I: Biopharmaceutics and Part II: Pharmacokinetics Module Course ECTS: 7 Course prerequisite/s: Physiology II and Pharmacology II Course Objective ▪ To develop the ability to logically apply the interrelationship of the physicochemical properties of the drug, the dosage form and ROA on the rate and extent of drug absorption. ▪ To develop a graduate with good practical knowledge and understanding of pk principles in everyday clinical pharmacy practice.
- 2. Introduction DRUG PRODUCT PERFORMANCE Drugs are substances intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease. Drugs are given in a variety of dosage forms or drug products such as solids, semisolids, liquids, etc, for systemic or local therapeutic activity. 12/14/2022 2
- 3. Introduction cont. 12/14/2022 3 • Drug products can be considered to be drug delivery systems if it release and deliver drug to the site of action such that they produce the desired therapeutic effect. • In addition, drug products are designed specifically to meet the patient’s needs including palatability, convenience, and safety which is a measure of drug product performance.
- 4. Introduction cont. Drug product performance is defined as the release of the drug substance from the drug product either for local drug action or for drug absorption into the plasma for systemic therapeutic activity. Advances in pharmaceutical technology and manufacturing have focused on developing quality drug products that are safer, more effective, and more convenient for the patient which is concerned by biopharmaceutics.. 12/14/2022 4
- 5. Biopharmaceutics In the world of drug development, the term “biopharmaceutics” narrowly defined as a field of science that involves the preparation, use, or dispensing of medicines (Woolf, 1981). Addition of the prefix “bio,” coming from the Greek “bios,” relating to living organisms or tissues expands this field into the science of preparing, using, and administering drugs to living organisms or tissues. 12/14/2022 5
- 6. Biopharmaceutics…cont’d Biopharmaceutics:- examines the interrelationship of the physical/chemical properties of the drug, the dosage form (drug product) in which the drug is given, and the route of administration on the rate and extent of systemic drug absorption. 12/14/2022 6
- 7. Biopharmaceutics…cont’d The importance of the drug substance and the drug formulation on absorption, and distribution of the drug to the site of action, is described as: A sequence of events that precede elicitation of a drug’s therapeutic effect. 12/14/2022 7
- 8. Figure 1: demonstrating the dynamic relationship between the drug, the drug product, and the pharmacologic effect. 12/14/2022 8
- 9. Cont.… • First, the drug in its dosage form is taken by the patient in any route of administration. • Next, the drug is released from the dosage form in a predictable and characterizable manner. • Then, some fraction of the drug is absorbed from the site of administration into either the surrounding tissue for local action or into the body (as with oral dosage forms), or both. Finally, the drug reaches the site of action 12/14/2022 9
- 10. Cont.… • A pharmacodynamic response results when the drug concentration at the site of action reaches or exceeds the minimum effective concentration (MEC). • The suggested dosing regimen, including starting dose, maintenance dose, dosage form, and dosing interval, is determined . 12/14/2022 10
- 12. Cont.… oFor a drug to be effective, enough of it needs to reach its site(s) of action and stay there long enough to be able to exert its pharmacological effect. oThis depends upon the route of administration, the form in which it is administered and the rate at which it is delivered. 12
- 13. Cont’d Generally, biopharmaceutics involves factors that influence 1. The design of the drug product, 2. Stability of the drug within the drug product, 3. The manufacture of the drug product 4. The release of the drug from the drug product, 5. The rate of dissolution/release of the drug at the absorption site, and 6. Delivery of drug to the site of action 12/14/2022 13
- 14. Concept of biopharmaceutics Factors affect (influence) bioavailability Food eaten by the patient Effect of disease state Age of the patient The site of administration Physicochemical properties of drug Co-administration of drug The type of dosage form, size & frequency 12/14/2022 14
- 15. Application of biopharmaceutics provides the scientific basis for drug product design and drug product development. • Each step in the manufacturing process to finished dosage form may potentially affect the release of the drug from the drug product and the availability of the drug at the site of action. Used to increase, monitor and maintain the rate at which a drug is released from the drug product 12/14/2022 15
- 16. Cont’d Used to characterize physiochemical nature of the drug rate of solubility and dissolution rate of the drug. 12/14/2022 16
- 17. Cont’d Relevant to preclinical, clinical, industrial pre- formulation and formulation development, as well as in regulatory affairs. It encompasses the field of characterizing, describing, evaluating, optimizing and predicting drug absorption, bioavailability and bioequivalency. 12/14/2022 17
- 18. Barriers of drug transport (epithelia and plasma membrane) Membranes are major structures in cells surrounding Functionally, cell membranes are semipermeable partitions that act as selective barriers to the passage of molecules. 12/14/2022 18
- 19. Cont’d Properties of the lipid membrane are critically important in regulating the movement of drug molecules. Other barrier properties of membranes are transport proteins and ion channels, Control the rate of permeation of many solutes. The relationship between membrane structure, membrane function, and cell physiology is basic determinant factor for drug transport. 12/14/2022 19
- 20. Cont’d Water, some selected small molecules, and lipid-soluble molecules pass through such membranes whereas highly charged molecules and large molecules, such as proteins and protein- bound drugs, do not pass. 12/14/2022 20
- 21. Mechanism of drug transport o For systemic drug absorption, the drug may have to cross cellular membranes o After oral administration, drug molecules must cross the intestinal epithelium by ▫ between the epithelial cells (paracellular absorption) ▫ going either through (transcellular absorption) or ▫ passive diffusion ▫ Carrier mediated transport ▫ Vascular transport 12/14/2022 21
- 22. Mechanism of drug transport…cont’d • 90 12/14/2022 22 1. Paracellular and transcellular routes, Paracellular transport is a passive transport process that occurs between adjacent epithelial cells. The rate-limiting step in this process is transport across the tight junction. This makes the tight junction the primary determinant of paracellular permeability.
- 23. Paracellular and transcellular routes…cont’d • However, two important qualifiers must be added to this simple statement. • First, an immutable characteristic of paracellular transport is that it is passive; therefore, it depends entirely on local concentration gradients. • Second, processes independent of tight junction regulation can affect mucosal permeability. • These is epithelial damage, including erosion or ulceration. 12/14/2022 23
- 24. Cont’d The paracellular transport is not suitable for the transport of large macromolecules. Several peptide drugs, such as octreotide, vasopressin analog desmopressin, and thyrotropin-releasing hormone are believed to absorb by this route (Pauletti et al., 1996). 12/14/2022 24
- 25. Transcellular route Transcellular conveyance the transference of solutes via a cell (Rhoades and Bell, 2012). The transcellular channel of transportation embraces transcellular diffusion or active carrier arbitrated transference. One typical instance is the passage of glucose from the intestinal lumen to the extracellular fluid by epithelial cells. 12/14/2022 25
- 26. Transcellular route…cont’d • Another example is naproxen against Alzheimer’s disease. • Overall, at least for polar drugs, it is likely, that the transcellular route provides the main pathway during percutaneous absorption 12/14/2022 26
- 27. 2. passive diffusion Passive diffusion is the process by which molecules spontaneously diffuse from a region of higher concentration to a region of lower concentration. If the drug has a low Mwt and is lipophilic, the lipid cell membrane is not a barrier to drug diffusion and absorption. 12/14/2022 27
- 28. Passive diffusion cont’d oDrug molecules move forward and back across a membrane. oWhen one side is higher in drug concentration, at any given time, the number of forward-moving drug molecules will be higher than the number of backward- moving molecules
- 29. Passive diffusion cont’d oPassive diffusion is the major absorption process for most drugs. oThe driving force for passive diffusion is higher drug concentrations on the mucosal side compared to the blood. oAccording to Fick's law of diffusion, drug molecules diffuse from a region of high drug concentration to a region of low drug concentration.
- 30. Passive diffusion cont’d where dQ/dt = rate of diffusion, D = diffusion coefficient, K = lipid water partition coefficient of drug in the biologic membrane A = surface area of membrane; h = membrane thickness, and C GI – C p = difference between the concentrations of drug in GIT and in the plasma.
- 31. Passive diffusion cont’d Different factors affect the rate of passive diffusion of drugs Concentration gradient If the drug is given orally, C Gl >> C p and a large concentration gradient is maintained, thus driving drug molecules into the plasma from the gastrointestinal tract.
- 32. Passive diffusion cont’d Partition coefficient, K, represents the lipid–water partitioning of a drug across the membrane in the mucosa. Drugs that are more lipid soluble have a larger value of K
- 33. Passive diffusion cont’d surface area, A, of the membrane Drugs may be absorbed from most areas of the gastrointestinal tract The duodenal area of the small intestine shows the most rapid drug absorption due to presence Folds of villi and microvilli.
- 34. Passive diffusion cont’d Thickness of membrane (h) Drugs usually diffuse very rapidly through capillary plasma membranes in the vascular compartments, in contrast to diffusion through plasma membranes of capillaries in the brain. In the brain, the capillaries are densely lined with glial cells, so a drug diffuses slowly into the brain as if a thick lipid membrane existed.
- 35. Passive diffusion cont’d Diffusion coefficient, D, Is a constant for each drug and is defined as the amount of a drug that diffuses across a membrane of a given unit area per unit time when the concentration gradient is unity The dimensions of D are area per unit time
- 36. Passive diffusion cont’d Because D, A, K, and h are constants under usual conditions for absorption, a combined constant P or permeability coefficient may be defined. C p << C GI If C p is negligible and P is substituted into fick’ law expression, the following relationship for Fick's law is obtained:
- 37. Passive diffusion cont’d oNote: The extravascular absorption of most drugs tends to be a first-order absorption process The rate of drug absorption is usually more rapid than the rate of drug elimination Due to the large concentration gradient i.e. C GI >> C p
- 38. 3. Carrier mediated transport Numerous specialized carrier-mediated transport systems are present in the body esp in the intestine for the absorption of ions and nutrients including drugs. Includes Active transport and Facilitated Diffusion
- 39. Carrier mediated transport…cont’d Carrier-mediated transport is a specialized process requiring a carrier that binds the drug to form a carrier–drug complex that shuttles the drug across the membrane.
- 40. Carrier mediated transport…cont’d Active Transport Active transport is a carrier-mediated transmembrane process that plays an important role GI absorption and in renal and biliary secretion of many drugs and metabolites A few lipid-insoluble drugs that resemble natural physiologic metabolites (such as 5-fluorouracil) are absorbed from GIT by active transport
- 41. Carrier mediated transport…cont’d Active transport is characterized by the transport of drug against a concentration gradient The carrier molecule may be highly selective for the drug molecule. If the drug structurally resembles a natural substrate that is actively transported, then it is likely to be actively transported by the same carrier mechanism. drugs of similar structure may compete for sites of adsorption on the carrier.
- 42. Carrier mediated transport…cont’d Facilitated Diffusion oFacilitated diffusion is also a carrier-mediated transport system, differing from active transport in that the drug moves along a concentration gradient. oThis system is: saturable, structurally selective for the drug and shows competition kinetics for drugs of similar structure play a very important role in drug absorption
- 43. Carrier mediated transport…cont’d Carrier-Mediated Intestinal Transport oVarious carrier-mediated systems (transporters) are present at the intestinal brush border and basolateral membrane for the absorption of specific ions and nutrients essential for the body. oMany drugs are absorbed by these carriers because of the structural similarity to natural substrates
- 44. Carrier mediated transport…cont’d A transmembrane protein, P-glycoprotein (Pgp) is found in the intestine. Pgp appears to reduce apparent intestinal epithelial cell permeability from lumen to blood for various lipophilic or cytotoxic drugs. Other transporters in the intestines E.g. many oral cephalosporins are absorbed through the amino acid transporter
- 45. Carrier mediated transport…cont’d Intestine transporters and examples of drugs transported 12/14/2022 45 Transporter Example of drugs Amino acid transporter Gabapentin Baclofen Methyldopa L-dopa Oligopeptide transporter Cefadroxil Cephradine Cefixime Ceftibuten Cephalexin Captopril Phosphate transporter Fostomycine Monocarboxylic acid transporter Salicylic acid Benzoic acid Pravastatin
- 46. 4. Vesicular Transport oVesicular transport is the process of engulfing particles or dissolved materials by the cell. oPinocytosis and phagocytosis are forms of vesicular transport that differ by the type of material ingested. oPinocytosis refers to the engulfment of small solutes or fluid ophagocytosis refers to the engulfment of larger particles or macromolecules generally by macrophages
- 47. Vesicular Transport…cont’d During pinocytosis or phagocytosis, the cell membrane invaginates to surround the material and then engulfs the material, incorporating it into the cell. Subsequently, the cell membrane containing the material forms a vesicle or vacuole within the cell.
- 48. Vesicular Transport…cont’d oVesicular transport is the proposed process for the absorption of orally administered Sabin polio vaccine and various large proteins. oAn example of exocytosis is the transport of a protein such as insulin from insulin-producing cells of the pancreas into the extracellular space.
- 49. A certain type of protein called transport protein may form an open channel across the lipid membrane of the cell. Very small molecules, such as urea, water and sugars are able to rapidly cross the cell membrane through these pores. 5- Pore (convective) transport:
- 50. Transport of Substances Across a Membrane by Channel Proteins
- 51. Factors affecting oral drug absorption oThe systemic absorption of a drug after oral absorption is dependent on Physiologic Factors the physicochemical factors Formulation factors oConsidering all of these factors are important in the manufacture and biopharmaceutic evaluation of drug products
- 52. GIT anatomy and physiology oThe GIT is a muscular tube approximately 6 m in length with varying diameters. oIt stretches from the mouth to the anus and consists of four main anatomical areas: the oesophagus, stomach, the small intestine and the large intestine or colon Physiologic Factors
- 54. GIT anatomy and physiology…cont’d The luminal surface of the tube is very rough This roughness : increase the surface area for absorption.
- 55. GIT anatomy and physiology…cont’d oThe wall of the GIT is essentially similar in structure along its length oconsists of four principal histological layers 1. Serosa 2. Muscularis externa 3. Submucosa 4. Mucosa
- 56. GIT anatomy and physiology…cont’d The majority of the GI epithelium is covered by a layer of mucus. Mucus is a viscoelastic translucent aqueous gel that is secreted throughout the GIT, Acting as a protective layer and a mechanical barrier. The layer is continuous in the stomach and duodenum, but may not be so in the rest of the small and large intestines Mucus is constantly being removed from the luminal surface of the GIT through abrasion and acidic and enzymatic breakdown
- 57. GIT anatomy and physiology…cont’d Oesophagus oThe mouth is the point of entry for most drugs (so called peroral - via the mouth - administration). At this point contact with the oral mucosa is usually brief. oLinking the oral cavity with the stomach is the oesophagus oThis is composed of a thick muscular layer approximately 250 mm long and 20 mm in diameter.
- 58. Oesophagus…cont’d It joins the stomach at the gastrooesophageal junction, or cardiac orifice Squamous epithelium of non-proliferative cells of the oesophagus is mainly used for protective function. • secrete mucus into the narrow lumen to lubricate food and protect the lower part of the oesophagus from gastric acid. • The PH ranges between 5 and 6
- 59. GIT anatomy and physiology…cont’d oThe two major functions of the stomach are: to act as a temporary reservoir for foods, drugs and other ingested materials and to deliver it to the duodenum at a controlled rate; oAnother less function of the stomach is its role in reducing the risk of noxious agents reaching the intestine. oIts opening to the duodenum is controlled by the pyloric sphincter. Stomach
- 60. Stomach…cont’d oThe stomach can be divided into four anatomical regions the fundus, the body, the antrum and the pylorus.
- 61. Stomach…cont’d The stomach has a capacity of app 1.5 L although under fasting conditions it usually contains NMT 50 mL of fluid (mostly gastric secretions) These include: acid secreted by the parietal cells, which maintains the pH of the stomach 1 - 3.5 in the fasted state The release of gastrin hormone, which is a potent stimulator of gastric acid production is stimulated by peptides, amino acids and distension of the stomach;
- 62. Stomach…cont’d pepsins, which are secreted by the peptic cells in the form of its precursor pepsinogen Pepsins are peptidases which break down proteins to peptides at low pH. Above pH 5 pepsin is denatured; Mucus, which is secreted by the surface mucosal cells and lines the gastric mucosa In the stomach the mucus protects the gastric mucosa from autodigestion by the pepsin-acid combination
- 63. Stomach…cont’d olittle drug absorption occurs in the stomach owing to its small surface area compared to the small intestine. oThe rate of gastric emptying can be a controlling factor in the onset of drug absorption from the major absorptive site, the small intestine.
- 64. GIT anatomy and physiology…cont’d • The small intestine is the longest (4-5 m) and most convoluted part of GIT, extending from the pyloric sphincter of the stomach to the ileocaecal junction where it joins the large intestine. • Its main functions are: Digestion Absorption Small intestine
- 65. Small intestine…cont’d oThe small intestine is divided into the duodenum, which is 200-300 mm in length the jejunum, which is approximately 2 m in length, and the ileum, which is approximately 3 m in length.
- 66. Small intestine…cont’d oThe wall of the small intestine has a rich network of both blood and lymphatic vessels. oMost of cardiac output flows through the gastrointestinal viscera. oThe blood vessels of the small intestine receive blood from the superior mesenteric artery via branched arterioles.
- 67. Small intestine…cont’d oThe blood leaving the small intestine flows into the hepatic portal vein, which carries it via the liver to the systemic circulation. oDrugs that are metabolized by the liver are degraded before they reach the systemic circulation. this is termed hepatic presystemic clearance, or first- pass metabolism.
- 68. Small intestine…cont’d The wall of the small intestine also contains lacteals, which contain lymph and are part of the lymphatic system. The lymphatic system is important in the absorption of fats and lipid character drugs from GIT. The luminal pH of the small intestine b/n 6 and 7.5
- 69. GIT anatomy and physiology…cont’d It stretches from the ileocaecal junction to the anus and makes app 1.5 m of the 6 m of GIT. It is composed of caecum (~85 mm in length), ascending colon (~200 mm), hepatic flexure, transverse colon (usually > 450 mm), splenic flexure, descending colon (~300 mm), sigmoid colon (~400 mm) and rectum Colon
- 70. Colon…cont’d oThe colon has no specialized villi. However, the microvilli of the absorptive epithelial cells, the presence of crypts, and the irregularly folded mucosae serve to increase the surface area of the colon. oThe SA of colon is approximately 1/30th that of SI
- 71. Colon…cont’d oThe main functions of the colon are: the absorption of sodium ions, chloride ions and water from the lumen in exchange for bicarbonate and potassium ions. Thus the colon has a significant homeostatic role in the body. the storage and compaction of faeces.
- 72. Colon…cont’d • The colon is permanently colonized by an extensive number (about 1012 per gram of contents) and variety of bacteria. This is capable of several metabolic reactions, including hydrolysis of fatty acid esters and the reduction of inactive conjugated drugs to their active form. the pH of the caecum is around 6-6.5.
- 73. Physiological factor…cont’d Blood flow: For most drugs, the optimum site for drug absorption after oral administration is the duodenum region The duodenal region is highly perfused with a network of capillaries which also gets high amount of bloods 12/14/2022 73
- 74. Physiological factor…cont’d GI Motility and gastric emptying time oGI motility tends to move the drug through the alimentary canal the drug may not stay at the absorption site oThe transit time of the drug in the GI tract depends on the physiochemical properties of the drug the type of dosage form, and various physiologic factors 12/14/2022 74
- 75. Physiological factor…cont’d Esophageal transit Most DFs transit esophagus in < 15 s Tablets/capsules taken in supine position are liable to lodge in esophagus esp. when taken without water Chance of adhesion depend on shape, size and type of formulation Delay in reaching stomach may delay drug's onset of action Cause damage or irritation to esophageal wall, e.g. KCl tablets
- 76. Physiological factor…cont’d Gastric emptying oGastric emptying time (GET) is time DFs take to traverse stomach oGET is highly variable Normal GET range b/n 5 min and 2 hrs GET over 12 hrs for large single units oGET depends on DF type Fed/fasted state of stomach
- 77. Physiological factor…cont’d oMany factors influence gastric emptying: DF (liquid vs. solid, unit DFs vs. multiparticulate DFs) presence and composition of food postural position drugs disease state
- 78. Physiological factor…cont’d oFactors delaying GET Fats and fatty acids in diet High viscosity of diet Lying on left side Diseases: depression, hypothyrodism, gastric ulcer Drugs: propantheline, atropine (antimuscarinic)
- 79. Physiological factor…cont’d oFactors promoting GET Fasting Lying on right side Diseases: anxiety, hyperthyrodism Drugs: Metoclopramide (antiemetic and gastroprokinetic)
- 80. Physiological factor…cont’d pre-systemic metabolism/ first-pass metabolism Is a phenomenon of drug metabolism whereby the concentration of a drug, is greatly reduced before it reaches the systemic circulation This first pass through the liver greatly reduce the bioavailability of the drug. Enzymes of the gastrointestinal lumen, gut wall enzymes, bacterial enzymes, and hepatic enzymes are responsible. 12/14/2022 80
- 82. Physiological factor…cont’d Gastrointestinal pH -The gastrointestinal pH may influence the absorption of drugs in a variety of ways: A- It may affect the chemical stability of the drug in the lumen e.g. penicillin G, erythromycin B- affect the drug dissolution or absorption e.g. weak electrolyte drug. 12/14/2022 82
- 83. Physiological factor…cont’d Disease state and physiological disorders: -Local diseases can cause alterations in gastric pH that can affect the stability , dissolution and absorption of the drug. -Partial or total gastrectomy results in drugs reaching the duodenum more rapidly than in normal individuals. -This may result in an increased overall rate of absorption of drugs that are absorbed in the small intestine. 12/14/2022 83
- 84. Physiological factor…cont’d Effect of Food: -The presence of food in the GIT can influence the rate and extent of absorption, via a range of mechanisms. A- Complexation • e.g.Tetracycline forms non-absorable complexes with calcium and iron, • advised patients not take products containing calcium or iron, such as milk, iron preparations.
- 85. Cont… B- Alteration of pH • Food tends to increase stomach pH. • This leads decrease the rate of dissolution and absorption of a weakly basic drug and increase that of a weakly acidic one. C- Alteration of gastric emptying • Fats and some drugs tend to reduce gastric emptying and thus delay the onset of action of certain drugs.
- 86. D- Stimulation of gastrointestinal secretions -GI secretions (e.g. pepsin) produced in response to food may result in the degradation of drugs that are susceptible to enzymatic metabolism. -Fats stimulate the secretion of bile. -Bile salts can increase the dissolution of poorly soluble drugs (griseofulvin). -can form insoluble and non-absorbable complexes with some drugs, such as neomycin and kanamycin.
- 87. E-Competition between food components and drugs for specialized absorption mechanisms • There is a possibility of competitive inhibition of drug absorption in case of drugs that have a chemical structure similar to nutrients required by the body for which specialized absorption mechanisms exist.
- 88. Cont… F-Increased viscosity of gastrointestinal contents The presence of food in the GIT provides a viscous environment which may result in: -Reduction in the rate of drug dissolution -Reduction in the rate of diffusion -Hence, there is reduction in drug bioavailability.
- 89. G- Food-induced changes in pre-systemic metabolism -Certain foods may increase the bioavailability of drugs that are susceptible to pre-systemic intestinal metabolism by interacting with the metabolic process. -E.g. Grapefruit juice is capable of inhibiting the intestinal cytochrome P450 (CYP3A) and thus taken with drugs that are susceptible to CYP3A metabolism which result in increase of their bioavailability.
- 90. H- Food-induced changes in blood flow • Food serve to increase the bioavailability of some drugs (e.g. propranolol) • Blood flow to the GIT and liver increases after a meal. 12/14/2022 90
- 91. II, Physical-Chemical Factors Physical-chemical factors affecting oral absorption include: A- pH-partition theory B- Lipid solubility of drugs C- Dissolution and pH D- Drug stability and hydrolysis in GIT E- Complexation F- Adsorption
- 92. A. pH - Partition Theory: -According to this theory, the GI epithelia acts as a lipid barrier for drugs absorbed by passive diffusion, and those that are lipid soluble will pass across the barrier. -The unionized form of weakly acidic or basic drugs (the lipid-soluble form) will pass across the GI epithelia, but impermeable to the ionized (poorly-lipid soluble) form of such drugs. -Consequently, the absorption of a weak electrolyte will be determined by the extent to which the drug exists in its unionized form.
- 93. B. Lipid solubility of drugs: -Some drugs are poorly absorbed after oral administration even though they are non-ionized in small intestine. - -Low lipid solubility of them may be the reason. -The best parameter to correlate between water and lipid solubility is partition coefficient.
- 94. Lipid solubility of drugs…cont’d Partition coefficient (p) = 𝑳 𝒄𝒐𝒏𝒄 𝑾 𝒄𝒐𝒏𝒄 where, [ L] conc is the concentration of the drug in lipid phase. [W] conc is the concentration of the drug in aqueous phase. -The higher p value, the more absorption is observed. 12/14/2022 94
- 95. C. Drug Dissolution: - Many drugs are given in solid dosage forms and therefore must dissolve before absorption can take place - If dissolution is the slow, it will be the rate determining step (the step controlling the overall rate of absorption) then factors affecting dissolution will control the overall process.
- 96. Drug Dissolution…cont’d -Drug dissolution is considered to be diffusion controlled process through a stagnant layer surrounding each solid particle. Diagram Representing Diffusion through the Stagnant Layer
- 97. Drug Dissolution…cont’d -Drug dissolution is described by the Noyes-Whitney equation: -Where D; diffusion coefficient, A: the surface area, Cs: the solubility of the drug, Cb con. of drug in the bulk solution, and h the thickness of the stagnant layer. -If Cb is much smaller than Cs then we have so-called "Sink Conditions" and the equation reduces to:
- 98. Factors affecting drug dissolution in the GIT: I Physiological factors: Diffusion coefficient, D: Presence food in the GIT increase the viscosity of the GI fluids reducing the rate of diffusion of the drug molecules away from the diffusion layer (↓ D) decrease in dissolution rate of a drug.
- 99. Cont’d Drug surface area, A: Surfactants increase the wettability of the drug which increase the drug solubility. The thickness of diffusion layer, h: An increase in GI motility decrease the thickness of diffusion layer around each drug particle, increase the dissolution rate of a drug.
- 100. II Physicochemical factors affecting the dissolution rate of drugs: Surface area, A: The smaller the particle size the greater the effective surface area of drug particle. Diffusion coefficient, D: The value of D depends on the size of the molecule and the viscosity of the dissolution medium. Solubility in the diffusion layer, Cs: The dissolution rate of a drug is directly proportional to its intrinsic solubility in the diffusion layer surrounding each dissolving drug particle
- 101. Drug Dissolution (cont.): Salts: Salts of weak acids and weak bases generally have much higher aqueous solubility than free acid or base. dissolution rate of a weakly acidic drug in gastric fluid (pH 1 – 3.5) will be relatively low. If the pH in the diffusion layer increased, the solubility, Cs, of the acidic drug in this layer, and hence its dissolution rate in gastric fluids would be increased.
- 102. Drug Dissolution (cont.): -pH of the diffusion layer will increased if the chemical nature of the weakly acidic drug changes from free acid to a basic salt (the sodium or potassium form of the free acid). -The pH of the diffusion layer would be higher (5-6) than the low bulk pH (1-3.5) of the gastric fluids because of the neutralizing action of the strong (Na+, K+ ) ions present in the diffusion layer. -The drug particles will dissolve at a faster rate and diffuse out of the diffusion layer into the bulk of the gastric fluid, where a lower bulk pH.
- 103. Drug Dissolution (cont.): -Thus the free acid form of the drug in solution, will precipitate out -This precipitated free acid will be in the form of: very fine, non-ionized, wetted particles, which have a very large surface area in contact with gastric fluids, facilitating rapid redissolution when additional gastric fluid is available.
- 104. Drug Dissolution (cont.): Dissolution process of a salt form of a weakly acidic drug in gastric fluid.
- 105. Drug Dissolution (cont.): -One example is the dissolution and bioavailability profiles of Penicillin V with various salts. These results might support the use of the benzathine or procaine salts for IM depot use and the potassium salt for better absorption orally.
- 106. Drug Dissolution (cont.): Crystal form: 1. Polymorphism: • Some drugs exist in a number of crystal forms or polymorphs. • These different forms may have different solubility. • Chloramphenicol palmitate is one example which exists in three crystalline forms A, B and C. A, is the stable polymorph B, is the metastable polymorph (most soluble) C, is the unstable polymorph
- 107. Drug Dissolution (cont.): 2. Amorphous solid: • The amorphous form dissolves more rapidly than the corresponding crystalline form. • For example, amorphous form of novobiocin antibiotic was readily absorbed following oral administration but, slowly converts to the more stable crystalline form, with loss of therapeutic effectiveness.
- 108. Drug Dissolution (cont.): Solvates: Solvates: If the drug is able to associate with solvent molecules to produce crystalline forms known as solvates. Hydrates: drug associates with water molecules.
- 109. Drug Dissolution (cont.): The greater the solvation of the crystal, the lower are the solubility and dissolution rate in a solvent identical to the solvation molecules The faster-dissolving anhydrous form of ampicillin was absorbed to a greater extent from hard gelatin capsules and an aqueous suspension than was the slower-dissolving trihydrate form.
- 110. D-Drug stability and hydrolysis in GIT: • Drugs that are susceptible to acidic or enzymatic hydrolysis in the GIT, suffer from reduced bioavailability. • Therefore, these drugs should be: Enteric coated Administration of chemical derivatives of the parent drug that exhibit limited solubility in gastric fluid, but liberate the drug in the small intestine.
- 111. E- Complexation: -Complexation of a drug may occur within the dosage form and/or in the GI fluids, and can be benefecial or deterimental to absorption. 1-Intestinal mucosa (mucin) + Streptomycin = poorly absorbed complex 2-Calcium + Tetracycline = poorly absorbed complex (Food-drug interaction)
- 112. F- Adsorption: • Certain insoluble substances may adsorbed co- administrated drugs leading to poor absorption. Charcoal (antidote in drug intoxication). Kaolin (antidiarrheal mixtures) Talc (in tablets as glidant)
- 113. III. Formulation Factors Affecting Oral Absorption: • The role of the drug formulation in the delivery of drug to the site of action should not be ignored. • Since a drug must be in solution to be absorbed efficiently from the G-I tract, you may expect the bioavailability of a drug to decrease in the order solution > suspension > capsule > tablet > coated tablet.
- 114. Formulation Factors…cont’d A.Solution dosage forms: - In most cases absorption from an oral solution is rapid and complete, compared with administration in any other oral dosage form. 12/14/2022 114
- 115. Formulation Factors…cont’d -Some drugs which are poorly soluble in water may be: 1-dissolved in mixed water/alcohol or glycerol solvents (cosolvency), 2- given in the form of a salt (in case of acidic drugs) 3-An oily emulsion or soft gelatin capsules have been used for some compounds with lower aqueous solubility to produce improved bioavailability.
- 116. Formulation Factors…cont’d B. Suspension dosage forms: • A well formulated suspension is second to a solution in terms of superior bioavailability. • A suspension of a finely divided powder will maximize the potential for rapid dissolution. • A good correlation can be seen for particle size and absorption rate. • The addition of a surface active agent will improve the absorption of very fine particle size suspensions.
- 117. Formulation Factors…cont’d C. Capsule dosage forms: • The hard gelatin shell should disrupt rapidly and allow the contents to be mixed with the G-I tract contents. • If a drug is hydrophobic a dispersing agent should be added to the capsule formulation • These diluents will work to disperse the powder, minimize aggregation and maximize the surface area of the powder.
- 118. Formulation Factors…cont’d D. Tablet dosage forms: Blood
- 119. Formulation Factors…cont’d The tablet is the most commonly used but, also quite complex in nature. 1-Ingredients Drug : may be poorly soluble, hydrophobic Lubricant : usually quite hydrophobic Granulating agent : tends to stick the ingredients together Filler: may interact with the drug, etc., Disintegration agent: break the tablet apart
- 121. What is Bioavailability? Drugs must cross several biological membranes to reach their sites of action. Extent and rate of penetration and hence systemic effect depends on ROA and absorption.
- 122. Cont’d Bioavailability: is a term that describes the rate and extent of drug absorption from a drug product and its availability at the site action. •Drug con. usually cannot be readily measured directly at the site of action. •Hence it is usually determined in the blood or urine •Bioavailability: how quickly and how much of a drug appears in the blood after a specific dose is administered.
- 123. Cont’d Bioavailable dose: The fraction of an administered dose of a particular drug that reaches the systemic circulation intact.
- 124. Factors Affecting Drug Bioavailability Physiologic Factors Related to Drug Absorption Passage of drug across cell membrane Gastric emptying rate and Transit time of drug in GIT Blood perfusion of the GIT, Intestinal motility Route of administration Variations in pH of GI fluids
- 125. Cont’d Interactions with other substances Pre-systemic and first-pass metabolism Exposure to various pH conditions, gut flora and enzymes Age, sex, weight, disease states of patient Physicochemical Nature of the Drug
- 126. Cont’d Nature of membrane The extent of ionization Fick’s law of diffusion •(dQ/dt =DAK/h (CGI – Cp) Interactions with other substances Fluid volume
- 127. Physicochemical Properties of the Drug •Drug bioavailability is also dependent on Permeability, Solubility and Dissolution rate In vivo, and Luminal degradation of the drug with in the body Fig 2.2: Dissolution and permeability of drug.
- 128. Pharmaceutic Factors Dosage Forms (DFD) Parenteral (e.g. IV, IM) Buccal: (Nitroglycerin sublingual tablet, fast dissolving excipient, better availability than oral, not affected by first- pass effect) Oral preparations: Solutions (elixirs, syrups, or simple solutions) generally result in faster and more complete absorption of drug, since a dissolution step is not required
- 129. Pharmaceutic Factors…cont’d Hard gelatin shell should disrupt rapidly and allow the contents to be mixed with the G-I tract contents Enteric-coated tablets, on the other hand, do not even begin to release the drug until the tablets empty from the stomach
- 130. Pharmaceutic Factors…cont’d Formulation Factors Tablets (additives) Filler: Type, amount, interaction with drug? Granulating/binding agent: (bonding) type, concentration, viscosity? Wetting agent: (+/-) Disintegrating agent: Amount, type Lubricant: Amount, type Special coatings: Film/enteric, amount/thickness
- 131. Pharmaceutic Factors…cont’d Manufacturing/Process Variables •Granulation: Method, blend uniformity, equipment (high shear mixers, fluidized bed) •Drying: Rate, Temp., equipment (tray, tunnel, fluidized bed), moisture level? •Coating method: Spraying, pouring, •Blending: Time, rate, equipment used •Compression force, dwell time, tooling used
- 132. Absolute and Relative Bioavailability Absolute Bioavailability (Fab) The systemic availability of an orally administered drug is calculated in comparison to its IV administration, it is called as absolute bioavailability. • It can be calculated by comparing the total amount of intact drug that reaches the systemic circulation following non- intravenous administration (e.g., oral, rectal, etc.) with its drug in systemic circulation following intravenous administration.
- 133. Absolute Bioavailability (Fab)…cont’d • Hence, absolute bioavailability of a drug is the systematic availability of a drug after extra vascular administration compared to intravenous administration. • There are different methods available to determine the extent of systemic availability. 12/14/2022 133
- 134. Absolute Bioavailability (Fab)…cont’d • One of the most commonly used methods is comparing AUC (area under the plasma drug concentration–time curve) after an intravenous and an extra vascular administration. • It ranges from F = 0 (no drug absorption) to F =1(complete drug absorption). 12/14/2022 134
- 135. Absolute Bioavailability (Fab)…cont’d Fig : AUC after IM and IV administration of same dose of a drug
- 136. Absolute Bioavailability (Fab)…cont’d • Extra vascular administration of the drug includes routes such as oral, rectal, nasal, subcutaneous, etc. • IV dose is used as a standard/reference to compare the systemic availability of the drug administered via different routes. • When a drug is administered IV, no absorption barriers to cross and hence, it is considered to be totally (100%) bioavailable. • Absolute bioavailability is expressed as percentage. 12/14/2022 136
- 138. Relative Bioavailability • When the systemic availability of a drug after administration is compared with that of standard of the same drug, it is known as relative bioavailability. • Relative bioavailability can be calculated by comparing the plasma drug concentration-time-curves (AUC - area under the curve) after the administration of two different formulations of the same compound (e.g. capsule vs. tablet). 12/14/2022 138
- 139. Relative Bioavailability…cont’d Thus, relative bioavailability is the systematic availability of the drug from a dosage form as compared to the reference standard given by the same route of administration. 12/14/2022 139
- 140. Relative Bioavailability…cont’d • A drug which cannot be administered through IV route, then instead of absolute bioavailability, the relative bioavailability can be calculated. • In this scenario, the bioavailability of a given drug is compared to that of the same drug administered in a standard dosage form (standard can be clinically proven preparation). • Relative bioavailability is expressed as percentage. 12/14/2022 140
- 141. Relative Bioavailability…cont’d Fig AUC after administration of same dose of Product A and Product B of same drug
- 143. Methods of Assessing Bioavailability • Assessment of Bioavailability is required for various purposes.
- 144. Methods of Assessing Bioavailability in vivo Bioavailability in vivo can be assessed by: Pharmacokinetic parameters and pharmacodynamic parameters: 12/14/2022 144
- 145. Pharmacokinetic parameters AUC (Area Under the Curve) • AUC is a measurement of the extent of drug bioavailability. • The AUC reflects the total amount of active drug that reaches the systemic circulation. • is expressed in mcg/ml * hours. Cmax (Peak plasma drug concentration) • It is the maximum plasma drug con. after oral admn of drug • Cmax is expressed in mcg/ml. Tmax (Time of peak plasma drug concentration) • The time required to reach maximum drug con, in plasma after extravascular drug administration. • Useful in estimating the rate of absorption & expressed in hours. 12/14/2022 145
- 146. Pharmacodynamic parameters Minimum Effective Concentration (MEC) / Minimum Inhibitory Concentration (MIC) • MEC/MIC is the minimum con, of drug in plasma required to produce the therapeutic effect. • The con, of drug below MEC is said to be in the sub‐therapeutic level. • MIC term is generally used in case of antibiotics and it describes the minimum concentration of antibiotic in plasma required to kill or inhibit the growth of micro-organisms. 12/14/2022 146
- 147. Pharmacodynamic parameters…cont’d Maximum Safe Concentration (MSC) / Maximum Safe Dose (MSD) • MSC/MSD is the con, of drug in plasma above which adverse or unwanted effects are expected to happen. • Concentration of drug above MSC is said to be in the toxic level. 12/14/2022 147
- 148. Pharmacodynamic parameters…cont’d Duration of action • The time period for which the plasma con, of drug remains above the MEC level is known as duration of (drug) action. • It can also be defined as the difference between onset time and time for the drug to drop back to MEC. Onset of action • When plasma drug concentration just exceeds the required MEC, the pharmacological response starts and this is called as onset of action. 12/14/2022 148
- 149. Pharmacodynamic parameters…cont’d Onset time • It is the time required by the drug to start producing pharmacological response. • It corresponds to the time for the plasma con, to reach (MEC) after administration of drug. Intensity of action (Peak response) • It is the maximum pharmacological response produced by the peak plasma concentration of drug. Therapeutic Range (Therapeutic window) • The con, of drug between MEC and MSC is known as therapeutic range. 12/14/2022 149
- 150. 12/14/2022 150
- 152. In -Vitro Approaches • Drug dissolution studies under a certain condition give an indication of drug bioavailability. • Dissolution studies are often the preferred method in several test formulations of the same drug. • The test formulation that demonstrates the most rapid rate of drug bioavailability in-vitro will generally have the most rapid rate of drug bioavailability in-vivo. 12/14/2022 152
- 153. In Vitro/ in Vivo Correlations (IVIVC) • An IVIVC is a predictive mathematical model that describes the relationship between an in vitro property of a dosage form and a relevant in vivo response. • When performing IVIVC for formulation development, the in vitro property is primarily dissolution or drug release and the in vivo response is primarily a drug’s plasma con,. 12/14/2022 153
- 154. Why Conduct IVIVC? • An IVIVC model is recommended by regulatory authorities for most modified release DF. • The main advantage of IVIVC is to provide a mechanism for evaluating the change in in vivo absorption based on in vitro dissolution changes when there are small changes in a formulation. 12/14/2022 154
- 155. Cont’d • Once a validated IVIVC model has been established, it can be used to predict bioavailability and bioequivalence (BA/BE) based on in vitro data that are already available • In such cases, dissolution test results can be used to provide the desired information without the need for any clinical BE studies with human subjects. 12/14/2022 155
- 156. Cont’d • Another advantage of IVIVC is that, it conveys a better understanding of the drug product itself. • This can help establish a wider drug product acceptance criteria and formulation stability. • IVIVC can also be especially useful for predicting the in vivo effects of changes to the formulation components, manufacturing site, or process. 12/14/2022 156
- 157. Cont’d • IVIVC is extremely important during initial product development. • Establishing an IVIVC model can be even more valuable after the product has been approved by determining the impact of post-approval manufacturing changes. • All of this can be determined without having to repeat costly in vivo BE studies. 12/14/2022 157
- 158. Benefits of IVIVC IVIVC analyses can be used to support: • Abbreviated New Drug Applications (ANDA) • New Drug Applications (NDA) for oral drugs with extended release characteristics • Abbreviated Antibiotic Drug Applications (AADA) as a surrogate for in vivo BE determinations 12/14/2022 158
- 159. Levels of IVIVC • There are three primary IVIVC categories, known as Levels A, B, and C. • There is also a subcategory known as multiple Level C correlation. • Level A is the most common type of IVIVC and historically used primarily for NDAs and investigational new drug (IND) applications. • Level C can be useful in the early stages of development and is the second most common. • Level B and Multiple Level C correlations are comparatively rare. 12/14/2022 159
- 160. Level A • Level A correlation is generally linear and represents a point- to-point relationship between in vitro dissolution rate and in vivo input rate. • Level A should be used when demonstrating an IVIVC relationship for two or more formulations with different release rates. • Correlation is usually estimated by a two-stage procedure: • deconvolution followed by comparison of the fraction of drug absorbed to the fraction of drug dissolved. 12/14/2022 160
- 161. Level A…cont’d In a linear correlation, the in vitro dissolution and in vivo input curves may be directly superimposable. fig:Fa versus Fd profile for diltiazem HCl ER capsules. release is rate-limiting, resulting in a linear profile (Level A)
- 162. Level B • Level B correlation uses the same data used in Level A, but is based on the principles of statistical moment analysis. The mean in vitro dissolution time of the drug is compared to either: The mean in vivo residence time or The mean in vivo dissolution time • Level B is the least useful for regulatory purposes because it does not reflect the actual in vivo plasma level curves.. 12/14/2022 162
- 163. Level C • Level C correlation involves determining the relationship between in vivo pharmacokinetic (PK) parameters (e.g. Cmax, AUC,) and in vitro dissolution data at a single point. • Level C can predict Cmax and AUC, which can help you to establish BA and BE. • But, Level C does not reflect the complete shape of the plasma con, time curve. 12/14/2022 163
- 164. Multiple Level C • Multiple Level C correlation relates one or several PK parameters of interest to the amount of drug dissolved at several time points and can be as beneficial as Level A correlation. 12/14/2022 164
- 165. Biopharmaceutical Classification System (BCS) BCS is an experimental model that measures permeability and solubility under prescribed conditions. The original purpose of the system was to aid in the regulation of post-approval changes and generics, providing approvals based solely on in vitro data when appropriate. Importantly, since the majority of drugs are orally dosed, the system was designed around oral drug delivery. 12/14/2022 165
- 166. BCS…cont’d • This system can be used to flag drugs that should not be tested clinically unless appropriate formulation strategies are employed. • For example, a BCS Class II compound permeable but relatively insoluble would likely not be a good clinical candidate without the use of enhanced formulation techniques aimed at increasing solubility or rate of dissolution. • BCS used as a tool in drug product development. 12/14/2022 166
- 168. Bioequivalence "two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent and Their bioavailabilities (rate and extent) after administration in the same molar dose are similar to such a degree that their effects, with respect to both efficacy and safety, can be expected to be essentially the same.
- 169. Bioequivalence…cont’d • Pharmaceutical equivalence implies the same amount of the same active substance(s), in the same dosage form, for the same route of administration and meeting the same or comparable standards." 12/14/2022 169
- 170. 12/14/2022 170 Fig: pharmaceutical equivalence of two drugs
- 171. Bioequivalence…cont’d •For a generic drug to be considered bioequivalent to a pioneer product, there must be no statistical differences (as specified in the accepted criteria) between their plasma concentration-time profiles •Because two products rarely exhibit absolutely identical profiles, some degree of difference must be considered acceptable.
- 172. Bioequivalence…cont’d Two dosage forms are not bioequivalent Two dosage forms are bioequivalent:
- 173. Therapeutic Equivalence & Related Terms Pharmaceutical equivalence Are drug products in identical DF and ROA that contain identical amounts of the identical active drug ingredient. Meet the identical compendial or other applicable standard of: 12/14/2022 173 • Identity • Strength • Quality • Purity • Potency • Content uniformity • Disintegration time • Dissolution rate
- 174. Cont’d Pharmaceutical Alternatives. Are drug products that contain the identical therapeutic moiety, or its precursor, but not necessarily in the same amount or dosage form, or the same salt or ester. • E.g. quinidine sulfate, 200mg tablets vs. quinidine sulfate, 200mg capsules Different dosage forms and strengths within a product line by a single manufacturer are pharmaceutical alternatives, 12/14/2022 174
- 175. Cont’d Therapeutic Equivalents. Approved drug products are considered to be therapeutic equivalents if they are pharmaceutical equivalents for which bioequivalence has been demonstrated, and they can be expected to have the same clinical effect and safety profile. 12/14/2022 175
- 176. Therapeutic Equivalence…cont’d Two products are considered to be "therapeutic equivalents" if they each meet the following criteria: 1. They are pharmaceutical equivalents, 2. they are bioequivalent (demonstrated either by a bioavailability measurement or an in vitro standard),
- 177. Therapeutic Equivalence…cont’d 3. They are in compliance with compendial standards for strength, quality, purity and identity, 4. They are adequately labeled, and 5. they have been manufactured in compliance with Good Manufacturing Practices as established by the FDA. 12/14/2022 177
Editor's Notes
- drug molecules move forward and back across a membrane. If the two sides have the same drug concentration, forward-moving drug molecules are balanced by molecules moving back, resulting in no net transfer of drug. When one side is higher in drug concentration, at any given time, the number of forward-moving drug molecules will be higher than the number of backward-moving molecules; the net result will be a transfer of molecules to the alternate side
- Because the drug distributes rapidly into a large volume after entering the blood, the concentration of drug in the blood initially will be quite low with respect to the concentration at the site of drug absorption. If the drug is given orally, then C Gl >> C p and a large concentration gradient is maintained, thus driving drug molecules into the plasma from the gastrointestinal tract.
- The thickness of the hypothetical model membrane, h, is a constant for any particular absorption site. Drugs usually diffuse very rapidly through capillary plasma membranes in the vascular compartments, in contrast to diffusion through plasma membranes of capillaries in the brain. In the brain, the capillaries are densely lined with glial cells, so a drug diffuses slowly into the brain as if a thick lipid membrane existed. The term blood–brain barrier is used to describe the poor diffusion of water-soluble molecules across capillary plasma membranes into the brain. However, in certain disease states such as meningitis these membranes may be disrupted or become more permeable to drug diffusion.
- This equation is expression for a first-order process.
- In the intestine, drugs and other molecules can go through the intestinal epithelial cells by either diffusion or a carrier-mediated mechanism. Numerous specialized carrier-mediated transport systems are present in the body, especially in the intestine for the absorption of ions and nutrients required by the body Includes Active transport and Facilitated Diffusion
- Active transport is a carrier-mediated transmembrane process that plays an important role in the gastrointestinal absorption and in renal and biliary secretion of many drugs and metabolites. A few lipid-insoluble drugs that resemble natural physiologic metabolites (such as 5-fluorouracil) are absorbed from the gastrointestinal tract by this process. Active transport is characterized by the transport of drug against a concentration gradient—that is, from regions of low drug concentrations to regions of high concentrations. Therefore, this is an energy-consuming system. In addition, active transport is a specialized process requiring a carrier that binds the drug to form a carrier–drug complex that shuttles the drug across the membrane and then dissociates the drug on the other side of the membrane
- Facilitated diffusion is also a carrier-mediated transport system, differing from active transport in that the drug moves along a concentration gradient Therefore, this system does not require energy input. However, because this system is carrier mediated it is saturable and structurally selective for the drug and shows competition kinetics for drugs of similar structure. In terms of drug absorption, facilitated diffusion seems to play a very minor role.
- A transmembrane protein, P-glycoprotein (Pgp) is found in the intestine. Pgp appears to reduce apparent intestinal epithelial cell permeability from lumen to blood for various lipophilic or cytotoxic drugs Other transporters are also present in the intestines. For example, many oral cephalosporins are absorbed through the amino acid transporter. Cefazolin, a parenteral-only cephalosporin, is not available orally because it cannot be absorbed to a significant degree through this mechanism.
- Endocytosis and exocytosis are the processes of moving specific macromolecules into and out of a cell, respectively
- Vesicular transport is the proposed process for the absorption of orally administered Sabin polio vaccine and various large proteins An example of exocytosis is the transport of a protein such as insulin from insulin-producing cells of the pancreas into the extracellular space. The insulin molecules are first packaged into intracellular vesicles, which then fuse with the plasma membrane to release the insulin outside the cell
- 1. The serosa, which is an outer layer of epithelium and supporting connective tissue; 2. The muscularis externa, which contains two layers of smooth muscle tissue, a thinner outer layer which is longitudinal in orientation, and a thicker inner layer, whose fibres are oriented in a circular pattern. Contractions of these muscles provide the forces for movement of gastrointestinal contents; 3. The submucosa, which is a connective tissue layer containing some secretory tissue and which is richly supplied with blood and lymphatic vessels. A network of nerve cells, known as the submucous plexus, is also located in this layer; 4. The mucosa, which is essentially composed of three layers, the muscularis mucosa, which can alter the local conformation of the mucosa, a layer of connective tissue known as the lamina propria, and the epithelium.
- The small intestine is the longest (4-5 m) and most convoluted part of the gastrointestinal tract, extending from the pyloric sphincter of the stomach to the ileocaecal junction where it joins the large intestine. Its main functions are: digestion: the process of enzymatic digestion, which began in the stomach, is completed in the small intestine. absorption: the small intestine is the region where most nutrients and other materials are absorbed.
- The ascending and descending colons are relatively fixed, as they are attached via the flexures and the caecum. The transverse and sigmoid colons, however, are much more flexible
- The colon is permanently colonized by an extensive number (about 1012 per gram of contents) and variety of bacteria. This large bacterial mass is capable of several metabolic reactions, including hydrolysis of fatty acid esters and the reduction of inactive conjugated drugs to their active form. The bacteria rely upon undigested polysaccharides in the diet and the carbohydrate components of secretions such as mucus for their carbon and energy sources. They degrade the polysaccharides to produce short-chain fatty acids (acetic, proprionic and butyric acids), which lower the luminal pH, and the gases hydrogen, carbon dioxide and methane. Thus the pH of the caecum is around 6-6.5. This increases to around 7-7.5 towards the distal parts of the colon. Recently there has been much interest in the exploitation of the enzymes produced by these bacteria with respect to targeted drug delivery to this region of the gastrointestinal tract.