Case record...Progressive supranuclear palsy
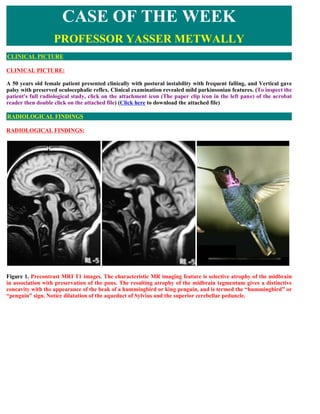
- 1. CLINICAL PICTURE: A 50 years old female patient presented clinically with postural instability with frequent falling, and Vertical gave palsy with preserved oculocephalic reflex. Clinical examination revealed mild parkinsonian features. (To inspect the patient's full radiological study, click on the attachment icon (The paper clip icon in the left pane) of the acrobat reader then double click on the attached file) (Click here to download the attached file) RADIOLOGICAL FINDINGS: Figure 1. Precontrast MRI T1 images. The characteristic MR imaging feature is selective atrophy of the midbrain in association with preservation of the pons. The resulting atrophy of the midbrain tegmentum gives a distinctive concavity with the appearance of the beak of a hummingbird or king penguin, and is termed the “hummingbird” or “penguin” sign. Notice dilatation of the aqueduct of Sylvius and the superior cerebellar peduncle. CASE OF THE WEEK PROFESSOR YASSER METWALLY CLINICAL PICTURE RADIOLOGICAL FINDINGS
- 2. Figure 2. MRI T1 images. Notice the posterior midbrain/uppermost pons signal changes representing astrogliosis in this region. Notice atrophy of the cerebellar cerebellar peduncle. Figure 3. MRI FLAIR images. On axial views, the selective atrophy of the midbrain tegmentum with relative preservation of the tectum and cerebral peduncles produces the “Mickey mouse” sign. Sometimes, the concavity of the lateral margin of the midbrain tegmentum is referred to as the “morning glory” sign and has high specificity but rather low sensitivity for PSP. Notice the periaqueductal signal changes representing astrogliosis. Also notice the dilatation of the aqueduct of Sylvius. Also notice evidence of central and cortical atrophy.
- 3. Figure 4. On axial views, the selective atrophy of the midbrain tegmentum with relative preservation of the tectum and cerebral peduncles produces the “Mickey mouse” sign (see Fig. 1B). Sometimes, the concavity of the lateral margin of the midbrain tegmentum is referred to as the “morning glory” sign and has high specificity but rather low sensitivity for PSP. Notice the periaqueductal signal changes representing astrogliosis. Also notice the dilatation of the aqueduct of Sylvius. Also notice evidence of central and cortical atrophy. MRI characteristics in progressive supranuclear palsy The characteristic MR imaging feature is selective atrophy of the midbrain in association with preservation of the pons. The resulting atrophy of the midbrain tegmentum gives a distinctive concavity with the appearance of the beak of a hummingbird or king penguin, and is termed the “hummingbird” or “penguin” sign. Periaqueductal signal changes (MRI T2 hyperintensities and MRI T1 hypointensity) representing astrogliosis in this region. Dilatation of the aqueduct of Sylvius. On axial views, the selective atrophy of the midbrain tegmentum with relative preservation of the tectum and cerebral peduncles produces the “Mickey mouse” sign. Sometimes, the concavity of the lateral margin of the midbrain tegmentum is referred to as the “morning glory” sign and has high specificity but rather low sensitivity for PSP. Non-specific changes such as central and cortical atrophy. DIAGNOSIS: PROGRESSIVE SUPRANUCLEAR PALSY (PSP) DISCUSSION: Progressive supranuclear palsy (PSP) is a degenerative neurological disorder of uncertain etiology characterized by gait ataxia, slowing or inability to generate voluntary saccadic eye movements, and axial rigidity. The most characteristic aspect of PSP is an inability to move the eyes, but the first symptom of PSP is usually unsteadiness and falling. PSP was first described as a distinct disorder in 1964. It is sometimes referred to as Steele-Richardson- DIAGNOSIS: DISCUSSION
- 4. Olszewksi, or "SRO", syndrome, from the names of the individuals who defined the disorder. Incidence PSP is estimated to affect about 4-6.4/100,000 persons, or about 5-6 percent of persons thought to have Parkinsonism. The incidence rate for new cases for ages 50-99 is 5.3/100,000, the crude incidence rate is 1.1/100K (Bower et al, 1997; Schrag et al, 1999). The peak incidence is in the early sixties. Men are affected slightly more often than women. PSP does not generally run in families, although there are some pedigrees reported. An illness resembling PSP is very common in Guam, sometimes in association with ALS (amyotrophic lateral sclerosis) and dementia. The cause of Guamanian PSP is unclear although it has been attributed to unusual dietary factors, possibly interaction with genetic factors (Cox and Sacks, 2002). A high prevalence of PSP like illness has been found on the island of Guadeloupe in the French West Indies (Caparros-Lefebre, 1999). In this population, it is suspected that a PSP like illness is related to ingestion of native teas called "soursop" and "sweetsop", both of which are forms of the "custard apple". A famous person with PSP was Dudley Moore, the actor. Clinical picture The symptoms and natural history of PSP was reported by Nath and others (Nath, Ben-Shlomo et al. 2003). The most frequent first symptom of PSP is several falls over a year. Next patients often develop some stiffness and at this point may be diagnosed as having "atypical Parkinsonism. However, patients with PSP rarely develop the resting tremor and stooped posture characteristic of Parkinsonism. As the disease progresses, most patients will develop problems controlling eye movement. Double vision is reported in more than half of all patients (Nath, Ben-Shlomo et al. 2003). The eye problems begin with vertical eye movements — patients may be unable to look downward. This may result in the so-called "dirty-tie" sign, because patients can’t see that they are dropping food when they eat. Difficulty in reading is common. Photophobia is reported in 43% (Nath, Ben-Shlomo et al. 2003). Eventually, patients lose the ability to look up and down at all, and usually about a year later, the ability to look from side-side is also lost. Eyelid apraxia occurs in 43% (Nath, Ben- Shlomo et al. 2003). Typically, patient with PSP have trouble controlling the sitting down process — they may "fall into their chair". There is an evidence for abnormal otoligh responses — small translational-VOR, and reduced VEMPs (Liao et al, 2008) Swallowing difficulties are also common in PSP (Litvan, 1997), the most common problem being delayed initiation of swallowing. The course of PSP was by many authors studied (Santacruz et al, 1998). There are a number of possible "signs" of PSP that will need confirmation. Ghika and Bogousslavski suggested that presymtomatic hypertension is a major feature in the diagnosis of PSP (1997). Aetiology It is known that the symptoms of PSP are caused by gradually progressive damage to a group of cells in a part of the brain called the "midbrain". These cells are involved in eye-movements and balance. The cause of the degeneration of these cells is unknown. In addition to the midbrain disease, there is also damage to the basal ganglia (especially globus pallidus), subthalamic nuclei, and the dentate nucleus of the cerebellum. According to Cordato et al (2000), atrophy of the basal ganglia is largely confined to the internal globus pallidus. Cerebral cortex is also affected and decreased metabolism of cerebral glucose correlates with dementia. Cortical benzodiazepine receptors are also decreased (Foster et al, 2000). Pathologically, gross examination of the brain in PSP shows midbrain atrophy. There is neuronal loss and neurofibrillary tangles in the basal ganglia, diencephalon and brainstem. The substantia nigra, subthalamic nucleus and pontine base are typically involved as well as the ventral anterior and lateral thalamic nuclei. The cerebellar dentate nucleus may show degeneration. Cortical pathology is minimal except for motor areas. See the review in Jellinger (1992) for more detail. The main suspicion for cause fell upon either a virus or a slow toxin. For example, a toxin called "MPTP", a contaminant in a drug of abuse, causes a condition similar to Parkinsonism. It has been speculated that there may be other slow-toxins in the environment, as for example, cycad nut or fruit bat consumption in Guam (Cox and Sacks, 2002) and certain herbal teas used in the Caribbean. With respect to the virus hypothesis, certain variants of
- 5. Parkinsonism are known to be related to strains of influenza, and it is conceivable that a so-far undescribed virus is the cause of PSP. Genetic studies, however, suggest that some cases of PSP is an autosomal recessive condition that maps to a polymorphism in the tau gene. (Bennet et al, 1998; Higgins et al, 1998-1999; Spillantini and Goedert, 2001). tau is a microtubule-binding protein that is normally abundant in neurons. There are six different forms of tau in normal human brain. In typical PSP, pathological tau is composed of aggregated 4-repeat (E10+) forms that accumulate in cells and glia in the brain (Searceant et al, 1999; Spillantini et al, 1998). Rojo et al (1999) reported 12 pedigrees with familial PSP. Relatives of patients with PSP tend to score more abnormally on screening tests for Parkinsonism (Baker and Montgomery, 2001), supporting either a genetic factor or exposure to a common environmental toxin. Genomic screens in persons with late-onset Parkinsons disease also suggests a linkage a mutation on the Tau gene on chromosome 17q (Martin et al, 2001) Delacourte et al suggested that tau is not the primary problem in most neural degenerations, but rather is a marker for vulnerable neurons that are damaged in several degenerative diseases (1998). Perhaps consistent with this line of logic, there are a multitude of other "tauopathies" including Alzheimer’s disease, Picks disease, ALS-Parkinson dementia complex of Guam, familial "tauopathy" (Murrel et al, 1997) , and Corticobasal degeneration (Higgins et al, 1999). There are four distinct kindreds with a mutation on chromosome 17 called N279K, and 50 kindreds having a syndrome called FTDP-17 for frontotemporal dementia and parkinsonism (Arima et al, 2000; Wszolek and Hutton, 2000), that lumps together 9 different mutations (Reed et al, 2000). The tau in PSP is different from that observed in Alzheimer’s disease and Picks, both in morphology and tau isoform content, but it resembles the tau in Corticobasal degeneration (Di Maria et al, 2000; Houlden et al, 2001) and FDTP-17. PSP certainly need not be entirely genetically determined — it also seems possible that PSP is partially controlled by genetic susceptability and also partially related to other stressors such as toxins or viruses. Oxidative stress, perhaps related to mitochondrial disorders, is another possibility. A PSP-like disorder has been reported after surgical repair of the ascending aorta (Mokri et al, 2004). This condition closely resembles PSP, but appears within weeks to months following this type of surgery. Pathologically, PSP is defined by the accumulation of neurofibrillary tangles in the brain. [2] Different rates and patterns of the accumulation of phosphorylated tau protein may account for the variation in clinical phenomena seen in patients with PSP. Liao and colleagues suggest that abnormal otolith-mediated reflexes may be at least partly responsible for the frequent falls in patients with PSP. They found that during near viewing, the translational vestibulo-ocular reflex responses in patients with PSP were, on average, only 12% of those of control subjects (p = 0.001). The amplitude of vestibular-evoked myogenic potentials was also significantly reduced in PSP patients compared with normal controls. [3] Diagnosis of PSP PSP is a clinical diagnosis, meaning that there is no absolute "test" for PSP. PSP is ordinarily diagnosed by a neurologist who has had experience with this condition. If anything, limitation or slowing of vertical saccades (see above) is the closest to a "litmus" test. Good recordings of vertical eye movements are not that easy, and to get this done properly requires good equipment It has been found that a particular type of MRI scan, Diffusion-weighting (DW), can differentiate PSP from Parkinsonism based on increased diffusion coefficients in the putamen. However, this type of MRI does not distinguish PSP from the Parkinson variant of multiple system atrophy (MSA). (Seppi, Schocke et al. 2003). In our opinion, while DW MRI should ideally be done as a confirmatory test when the PSP patient presents, there is little clinical usefulness in the DW scan as there are no effective treatments for PSP or MSA, and differentiation of PSP from Parkinsonism is not difficult for an experienced clinician. The superior cerebellar peduncle is also, on average, 20% smaller in PSP than in controls (Paviour et al, 2005). Practically this would seem to be a difficult distinction to make in clinical material. The ratio of the area of the midbrain to the pons on saggital MRI has reported to be an accurate method of detecting PSP (Oba and others, 2005), as the midbrain is smaller in PSP. This method seems promising.
- 6. Figure 5. Midsagittal T1-weighted MR images in a patient with PD (A), a patient with Parkinson variant of MSA (MSA-P) (B), and a patient with PSP (C). (A) There is no pontine or midbrain atrophy in the patient with PD. (B) Pontine atrophy (arrow) without midbrain atrophy in the Parkinson variant of MSA (MSA-P) patient. (C) Midbrain atrophy without pontine atrophy (divided by the white line) in the PSP patient, forming the silhouette of the “penguin” or “hummingbird” sign, with the shapes of midbrain tegmentum (bird’s head; above the white line) and pons (bird’s body; below the white line) looking like the lateral view of a standing penguin (especially the king penguin) or hummingbird, with a small head and big body. Another approach is a spinal fluid test developed in Italy for "trunacted Tau forms" (Borroni et al, 2008). This spinal fluid test, not currently available at a clinical test, was reported as extremely sensitive and specific for PSP. As there is no commercial method of doing this test, it’s clinical utility is presently absent. There are several very obscure neurological diseases that can be confused with PSP (Table 1), and it is wise to seek out a "tertiary care" neurologist who is familiar with PSP. This usually entails being referred to a "movement disorders clinic" in a university hospital setting. Experienced neurologists are generally accurate in making the diagnosis of PSP, being "right" about 90% of the time. General practitioners often misdiagnose PSP as Parkinsonism (Nath, Ben-Shlomo et al. 2003). Eye movements, particularly saccades are nearly always abnormal, but there are other (rare) causes of slowed eye movements. Classically it is taught that in PSP there is an "axial rigidity", meaning that the limbs may be relatively normal while the neck and trunk are rigid. This idea was called into question by a paper by Tanigawa that suggested that only the neck is rigid (Tanagawa et al, 1998). There are some unusual tests that may be positive, such as the "applause sign" — see next section. Blood tests, CT and MRI scans are usually normal. Tilt table and Valsalva testing may be abnormal in PSP as well as related disorders such as MSA. (Schmidt et al, 2009). A large number of other radiological techniques have been suggested (Golbe, 2004). Several of these depend on documenting subtle shrinkage of the midbrain and related areas using MRI (Paviour et al, 2005). Other reported methods include differences in the amplitudes of transcranial magnetic stimulation, and SPECT scan. At this writing (6/2005), we feel that these methods are of no practical utility and also need confirmation.
- 7. Figure 6. (A) Sagittal T1-MR imaging demonstrates volume loss in the midbrain with relative preservation of the pons. The midbrain tegmentum has lost its normal convexity giving it the appearance of a hummingbird (or penguin), also known as the “hummingbird sign.” (B) T2-weighted axial MR imaging demonstrates “Mickey mouse” or “morning glory” sign with concavity of the lateral margin of midbrain tegmentum. The first clinicopathologic descriptions of PSP were published in 1963 and 1964 and proved to be remarkably accurate.9 Only in the past 15 years have neurologists and basic scientists again focused on this disorder. The onset of PSP is insidious, and usually a prolonged phase of vague fatigue, headaches, arthralgias, dizziness, and depression occurs. Patients also develop subtle personality changes, memory problems, and pseudobulbar symptoms, and family members are often a more accurate source of such information than the patient. The initial symptoms can often involve unexplained imbalance or falls. Over time, dysarthria, dysphagia, and visual symptoms ensue.
- 8. In a neuropathologic study, the most common symptoms at disease onset were postural instability and falls (63%); dysarthria (35%); bradykinesia (13%); and visual disturbances such as diplopia, blurred vision, burning eyes, and light sensitivity (13%). [10] The cardinal manifestations of PSP are supranuclear ophthalmoplegia; pseudobulbar palsy; prominent neck dystonia; parkinsonism; behavioral, cognitive, and gait disturbances that cause imbalance; and frequent falls. Although presentations vary and early predominance of a particular symptom is not unusual, a greater spectrum of symptoms inevitably ensues over time. Several other features have been reported, including sleep disturbance with insomnia, clumsiness, impaired handwriting, and oscillopsia. Although the full constellation of symptoms occurring in a progressive fashion over time is characteristic, the vertical gaze palsy is the most distinctive single clinical feature. Other features that can be prominent include focal or segmental dystonia in the form of limb dystonia or blepharospasm. [11] Patients can also have asymmetric apraxia resembling Corticobasal degeneration. [12] Micturition disturbances, including urinary incontinence, are common in the later stages. [13,14] A subset of patients present with a progressive apraxia of speech, nonfluent aphasia, or a combination of the two. [15] The physical examination emphasizes the clinical features previously outlined. PSP is characterized primarily by motor, cognitive, and visual symptoms. Documentation of cognitive function with attention to executive function is important. The cranial nerve examination should include detailed analysis of ocular motility as follows: Slow vertical saccades and square wave jerks are early signs in most patients. The classic gaze palsy in PSP is supranuclear ophthalmoplegia. Supranuclear in this context refers to a lesion above the ocular motor nuclei, thus sparing the ocular motor nuclei, nerve fascicles, and neuromuscular junctional and extraocular muscles. Examination features serve to establish that the infranuclear structures are intact and that the lesion lies within the supranuclear domain. A supranuclear vertical gaze limitation is improved following extravolitional pathway activation, such as the vestibular ocular reflex (VOR) or the Bell phenomenon. The Bell phenomenon consists of upward eye deviation behind closed lids. This can be assessed clinically by partially holding the eyelid open and instructing the patient to try forcefully closing the eye. The vertical VOR can be activated by manually flexing and extending the neck while the patient views a distant target. If the extent of the vertical eye movement limitation is improved with either of these maneuvers, then the lesion is supranuclear in origin. Figure 7. MRI T1 image showing midbrain atrophy with dilatation of the aqueduct in a case with PSP
- 9. Measurement of ocular alignment in the cardinal positions of gaze at near and distance viewing often discloses the source of any diplopic symptoms. Examination of the eyelid position and movements may yield critical information. The characteristic facies, especially when associated with dysarthria, may provide a nearly pathognomonic clinical picture. Examination of pursuit movements and the extent of ocular rotations are important. Often, the earliest symptoms relate to imbalance and dysarthria. The imbalance is part of an extrapyramidal syndrome that is inclusive of poor postural reflexes, axial greater than appendicular rigidity, and dysarthria (monotone with slight hypophonic quality). Resting tremor is unusual. The early appearance of gait and balance dysfunction is in contrast to the course of idiopathic Parkinson disease, in which imbalance tends to occur late in the disease. The gait in individuals with PSP tends to be wider based and unstable; these individuals have a tendency to fall in any direction because of impaired postural reflexes. Bradykinesia with masked facies and a startled expression are frequent findings. Retrocollis may be present; with lid retraction, it enhances the astonished, worried appearance. Increased rigidity without cogwheeling or tremor completes the motor picture. Visual symptoms tend to be a relatively early finding, but they may not be present at onset; rarely, they are absent entirely. The earliest eye sign often is slowing of vertical saccades and fast phases. Later, the classic vertical supranuclear ophthalmoparesis occurs; this typically involves downgaze before upgaze. As a supranuclear process, vertical eye movements can still be generated by the VOR until late in the course of the disease, although the Bell phenomenon is usually absent (supraduction with eye closure). Later in the disease course, this ophthalmoparesis affects horizontal, in addition to vertical, eye movements. Complete ophthalmoparesis may ensue late in the course. Additionally, nearly continuous square wave jerks are commonly observed with fixation. Square wave jerks consist of small (<5°) horizontal movements that take the eyes conjugately off target and then return the eye to the target after a brief 180- to 200-millisecond latency. Although occasional square wave jerks are a common finding in elderly individuals and may be normal if unaccompanied by other symptoms, more continuous square wave jerks are often associated with an underlying CNS disease. Convergence eye movements are often impaired, and convergence insufficiency may produce episodic diplopia at near distances. Impaired binocular fusional capacity may produce diplopia related to decompensated phorias. Impaired VOR suppression has also been noted. Several eyelid signs frequently occur in individuals with PSP, including lid retraction, eyelid opening or closing apraxia, blepharospasm, or lid lag. Loss of the fast component of the optokinetic nystagmus can precede gaze palsy. Cognitive dysfunction and personality change are common, but they are generally milder in degree compared to primary dementing illnesses such as Alzheimer disease. Slowed cognitive processing, sequencing and planning difficulties, mild memory difficulty, and apathy are typical. These are generally more prominent later in the disease course. Litvan and Mega et al discussed the neuropsychiatric aspects of PSP in greater detail. [16] The investigators administered the Neuropsychiatric Inventory (NPI) to 22 patients with PSP, 50 patients with Alzheimer disease, and 40 controls. The NPI focuses on the presence of delusions, hallucinations, agitation, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability, and abnormal motor behavior. The presence of high apathy scores coupled with low agitation and anxiety scale scores was used to correctly
- 10. identify patients with PSP 85% of the time. Litvan and Agid et al tested the accuracy of 4 proposed clinical diagnostic criteria for PSP. [17,18] These authors applied the proposed diagnostic criteria to autopsy-proven cases, including 24 cases of PSP, 29 cases of Lewy body disease, 10 cases of cortical-basal ganglionic degeneration, 7 cases of postencephalitic parkinsonism, 16 cases of multiple system atrophy, 7 cases of Pick disease, and 12 cases of other parkinsonian or dementing illnesses. None of the criteria demonstrated both high sensitivity and high predictive value. A regression analysis approach revealed that vertical supranuclear palsy with downgaze abnormalities and postural instability with unexplained falls were the most useful diagnostic features. A progressive disease course including these features constituted the mandatory inclusion criteria. Mandatory exclusion criteria included a history of encephalitis, hallucinations, cerebellar signs, noniatrogenic dysautonomia, unilateral dystonia, alien hand syndrome, early cortical dementia, or focal lesions on examination or imaging. These criteria performed better than previously published guidelines, with a mean sensitivity of 57% and positive predictive value of 85%. When applied to data from the patient’s last visit to clinic, the criteria revealed a sensitivity of 66% and positive predictive value of 76%. The participants in a National Institute of Neurological Disorders and Stroke (NINDS)/Society for PSP conference formulated clinical research criteria for the diagnosis of PSP. [19] They based these new criteria on literature review and then validated them using a clinical data set from autopsy-confirmed cases of PSP. Criteria for possible PSP are as follows: Criteria for probable PSP are vertical supranuclear palsy with prominent postural instability, falls in the first year of onset, and other features of possible PSP, as follows: Criteria for definite PSP are as follows: The proposed criteria for possible PSP are highly sensitive, while the criteria for probable PSP are highly specific, rendering each useful for different analyses and studies. These attempts at clinical diagnosis will hopefully be supplanted by a reliable and objective diagnostic test in the future. The presence of prominent cerebellar signs, hallucinations or dysautonomia in the absence of drug effect, early cortical dementia features, or unilateral dystonia casts doubt on the diagnosis of PSP and should prompt Gradually progressive disorder with onset when the individual is aged 40 years or older Either vertical supranuclear palsy or both slowing of vertical saccades and prominent postural instability with falls in the first year of onset No evidence of other diseases that can explain the clinical features Symmetric proximal greater than distal akinesia or rigidity Abnormal neck posture, especially retrocollis Poor or absent response of parkinsonism to levodopa therapy Early dysphagia and dysarthria Early cognitive impairment with at least 2 of the following: apathy, abstract thought impairment, decreased verbal fluency, imitation behavior, or frontal release signs History of probable or possible PSP and Histopathologic evidence that is typical of the disease
- 11. consideration of other neurodegenerative conditions. Differential diagnosis All patients with PSP have abnormal vertical eye movements. Patients with PSP rarely have tremor and the stooped posture characteristic of Parkinson’s disease. Another degenerative disease, Gaucher’s type-III, a metabolic storage disese, also causes a progressive supranuclear palsy, but it begins horizontally. Other disorders that may be mistaken for PSP include Corticobasal degeneration, Picks disease, multisystem atrophy (MSA) and diffuse Lewy body disease. There is also a described variant of myotonic dystrophy (MD2), which has some resemblence to PSP. MD2 affects tau (Maurage et al, 2005). A familial "tauopathy" with dementia closely resembling PSP was described (Murrell et al, 1997). Jacob-Creutzfeld disease and related disorders can also present with ataxia and a supranuclear gaze palsy, but the course is generally more rapid and dementia is more severe than in PSP. MRI may be able to differentiate PSP from Parkinsonism. (Oba and others, 2005) as the midbrain is smaller relative to the pons in PSP than in PD. Several simple clinical tests have been reported as being commonly abnormal in PSP, but rarely normal in Parkinsons disease(Kuniyoshi et al, 2002). These include a lack of habituation of blinking after a penlight is flashed 7 times in one eye, a sustained head deviation after unidirectional rotation in an office chair for 45 seconds, and the "applause" sign — the patient is asked to clap exactly three times, sees it demonstrated, but when they perform the test the clapping continues more than 3 times. Of these, the "applause sign" seems most potentially useful, with the head deviation sign also being a reasonable possibility. Litvan and others (1997) investigated features that differentiate PSP from these other disorders, as gone over in a table adapted and amended form their paper below. While not to be relied upon as absolute criteria, this analysis does point out that gait instability and supranuclear gaze abnormalities are key differentiating features. A syndrome superficially resembling PSP was reported in stiff-person syndrome (Oskarsson et al, 2008). Like PSP, this single patient had a vertical supranuclear gaze palsy. Unlike PSP, their saccadic performance fatigued with continuous testing (for 340+ seconds). This patient also developed a tremor. This is an interesting observation. As it is not possible to test most patients for 340 seconds, and in fact most patients with PSP have no oculomotor testing at all, it may be worth checking for GAD antibodies in a prospective fashion in patients carrying the diagnosis of PSP. Table 1. Differential diagnosis of PSP
- 12. Prognosis of PSP Patients with PSP all progress and the usual life span after diagnosis is 5 years (Bower et al, 1997). The median time from disease onset to first key motor impairment is 4 years, usually 2 years after initial consultation (Goetz, Leurgans et al. 2003). Death does not result as a direct effect of the disease but rather from complications such as pneumonia or pulmonary embolism, which may result from inability of the patients to move about and care for themselves. Of course, falling is common in persons with PSP that are still ambulatory. Unintelligible speech occurs at a median disease duration of 57 months, or about 5 years. (Goetz, Leurgans et al. 2003) When persons with PSP begin to cough after every meal, this generally indicates that there is considerable danger of pneumonia from aspiration, and a decision needs to be made whether or not to put in a feeding tube. Older age at onset and classification as probable PSP are factors associated with poorer survival. Early problems with falls, speech, swallowing, diplopia and early insertion of a gastrostomy tube predicts reduced survival (Nath, Ben-Shlomo et al. 2003) Treatment of PSP In a word, no treatment. In particular, there is no drug known that will stop or reverse the usual inexorable progression of the disease. Most neurologists will try using drugs for Parkinsonism, such as Sinemet. However, such drugs are mildly helpful in only about 50% of persons with PSP. As disability in PSP is due to neuronal damage, and neurons do not regenerate, stopping progression is presently the main goal of treatment. Logically, it would seem to us and others (Schneider and Mandelkow, 2008) that PSP is caused by tau, and that symptomatic medication is bound to fail. There simply is no reasonable method of regrowing neurons. Thus, prevention of progression should be the goal, and in particular, attention should be directed towards treatments that reduce tau. There are numerous supportive treatments: We recommend that patients with PSP be seen every 3-6 months by their neurologist, accompanied by their caregiver. Studies of specific symptomatic medications include: Pramipexole (Mirapex): Weiner et al (1999) reported no effect. Idazoxan: Cole et al (1994). 5/9 responded Zolpidem (Ambien). Moro et a (1999)l. This study reported transient improvement (about 2 hours). Physiostigmine. Fratelli et al. This cholinergic agonist had no effect on swallowing. Donepezil (Litvan, et al. 2001). Not recommended Riluzole. (Bensimon et al. 2009).) no effect. Co-Q. (Stamelou, 2008). small positive effects. Physical therapy (Zampieri and Di Fabio (2008). small positive effects Lithium. There are ongoing studies.
- 13. Patients and caregivers should be counseled regarding the usual course of the disease, so that they can plan to deal with gradually increasing disability and also clearly establish to their family their wishes in the event that they should not be able to communicate this in the future. It should be clear what the patient wishes about end of life care — should they be resussitated ? Intubated ? A stomach tube placed ? Driving privileges should be carefully evaluated as many patients become unsafe due to slow reactions and poor vision. Eye care: (adapted from Biusse et al, 2004) Medications that reduce accommodation or tearing should be carefully considered Punctal (tear duct) occlusion and artificial tears should be considered in patients with dry eyes Patients with tremor may benefit from use of a music stand or cookbook stand to read Patients should have good lighting. Reading glasses may require base in prisms (because of the lack of convergence) Monocular occlusion during reading may be considered. Avoidance of bifocals and progressive lenses may be helpful (in favor of several sets of single-vision glasses) Astigmatic correction in spectacles may be less preferable in persons with tremor or falls. Patients should use a finger to draw the eyes across a page if they have slow saccades. Blepharospasm and apraxia of eyelid opening can be treated with botulinum toxin. Management of PSP Some neurologists prescribe a medication in PSP patients called "seligiline", which was at one point thought to prevent progression in Parkinsonism. Seligiline has not been formally studied in PSP and it is presently unclear whether or not it is helpful. There is some evidence that this drug may inhibit apoptosis (programmed cell death), which might be helpful in PSP. When combined with L-dopa in patients with parkinsonism, this drug is associated with increased mortality (Katzenschlager et al, 2008). It is our view that this drug is worth trying, although not in combination with L-dopa. It is the authors impression, based on use of this drug in several patients, that seligiline reduces the rate of progression in PSP. Some PSP patients on low dose seligiline develop blood pressure instabilities, which limits its use. Ropinirole and pramipexole have both demonstrated a reduction in the rate of loss of nigrostriatal innervation as determined by imaging in PD patients, when compared with levodopa. Yamamoto, M. and A. H. Schapira (2008). While this is not the same as showing a neuroprotective effect (as the control was levodopa), nevertheless these agents are worth considering. Physical therapy has been reported by several groups to have a positive effect (Suteerawattananon et al, 2002; Zampieri and Di Fabio 2008). In general, while we favor physical therapy, it is unreasonable to expect that it will reverse the ongoing degenerative neurological processes that cause the clinical picture of PSP. There is a small literature that documents a positive effect of amitriptyline (Elavil), which is an antidepressant medication. (Engel 1996). This medication is, however, sedating, drys the eyes, and may also have very adverse effects on thinking.
- 14. Drugs like the calcium channel blockers may be also worth trying as they may prevent apoptosis (programmed cell death) as mentioned above for Seligiline. In Mice, nimodipine has been reported as "efficient" in a model for ALS, a related neurodegenerative disorder (Kriz et al, 2003). Minocycline, a tetracycline type antibiotic, has been shown to be neuroprotective in animal models of stroke, multiple sclerosis, Parkinson’s disease and Huntington’s disease (Arvin et al, 2002). A "cocktail" of minocycline, riluzole (a glutamate antagonist used in ALS) and nimodipine (a calcium channel blocker) was reported as "efficient" in mice in a model of ALS, another degenerative neurological disorder (Kris et al, 2003). Memantine (Namendia) is a newly released glutamate antagonist that may also be worth considering. We know of no studies of these drugs in PSP, however. Flupirtine, an analgesic agent not available in the USA, has several neuroprotective actions including anti-oxident, anti-glutamate, and anti-apoptotic actions. It has been found helpful in Creutzfeldt-Jakob disease, which is a degenerative neurological disease similar to "mad cow" disease (Otto M, et al). To our knowlege, it has not been tried in PSP. Vitamin E may be slightly helpful. In other degenerative neurological disorders, Vitamin E often has a small effect in reducing the rate of progression. It seems possible that agents that reduce oxidative stress (mainly vitamins) might slow the rate of progression in PSP. The "statin" drugs, used to lower cholesteral also protect against Alzheimer’s disease and provide neuroprotection (Chen et al, 2003). These drugs might reduce the speed of progression in PSP. We know of no study of this family of drugs in PSP. Other measures Be prepared Most individuals with PSP and their caregivers attempt to make realistic plans anticipating a slow neurological decline. Patients and caregivers should establish early on their wishes regarding invasive supportive care — intubation, feeding tubes — as these issues are almost certain to come up in the course of the disease. Support research As even though several thousand papers have been written about PSP, there is no treatment as yet, efforts to encourage research into its diagnosis, mechanism and treatment seem highly worthwhile. Give of yourself Patients with PSP should consider donating their brains for autopsy examination at the time of death. In this way, a cure for this condition may eventually be found. SUMMARY Progressive supranuclear palsy (PSP), also known as Steele-Richardson-Olszewski syndrome, is a neurodegenerative disease that affects cognition, eye movements, and posture. PSP was first described as a clinicopathologic entity in 1964. Characteristics include supranuclear, primarily vertical, gaze dysfunction accompanied by extrapyramidal symptoms and cognitive dysfunction. The disease usually develops after the sixth SUMMARY
- 15. decade of life, and the diagnosis is purely clinical. Currently, no proven effective therapy exists. The cause of PSP remains unknown. Most cases appear to be sporadic. Both environmental and genetic influences have been postulated. PSP is usually fatal within approximately 6 years of onset, with a range of 2-17 years, based on cohort patients dying under surveillance; life table analysis among Golbe’s entire cohort [13,14,15,16] revealed a median disease duration of 9.7 years. Conflicting reports exist regarding the influence of age at diagnosis on survival; Younger patients usaully survive longer, although this is not a uniform finding among other studies [13,36] The primary causes of death in patients with PSP are infections and pulmonary complications (eg, pneumonia) that are frequently related to immobility. Often, the primary morbidity relates to imbalance leading to immobility, although dementia, visual symptoms, and dysphagia are major concerns. Approximately 50% of patients with PSP require some aid to walk within 3 years of the initial symptoms. The interval from initial symptom occurrence to the need for a cane or a walker is 3.1 years, and the interval to confinement to a chair or bed is 8.2 years. Neuroimaging This elderly man had an atypical parkinsonian syndrome characterized by early postural instability and falls backward, a vertical supranuclear gaze palsy, axial rigidity, pseudobulbar palsy, frontal lobe signs, and a poor response to L-dopa. These are characteristic features of progressive supranuclear palsy (PSP), also known as Richardson disease. It has a prevalence of 5 per 100,000, but is commonly underdiagnosed. The clinical features are quite different from PD or other atypical parkinsonian syndromes such as MSA. However, there is a subgroup of progressive supranuclear palsy (PSP), patients, known as PSP-Parkinsonism (PSP-P), which presents with asymmetrical bradykinesia, jerky tremor, and an initial L-dopa response without vertical gaze palsy. Other unusual presenting features of progressive supranuclear palsy (PSP), include primary gait freezing, early frontotemporal dementia, and corticobasal syndrome. MR imaging of the brain can be normal in the early stages of disease. Nevertheless, certain MR imaging features can greatly assist in making the diagnosis especially in patients with PSP-P or an atypical presentation (Fig. 6). The first radiological clue for progressive supranuclear palsy (PSP), would be the presence of striking hyperextension of the neck on sagittal MR imaging. The characteristic MR imaging feature is selective atrophy of the midbrain in association with preservation of the pons. The resulting atrophy of the midbrain tegmentum gives a distinctive concavity with the appearance of the beak of a hummingbird or king penguin, and is termed the “hummingbird” [48] or “penguin” sign (see Fig. 6A). [49] Quantitative measurements of midbrain atrophy have been shown to improve diagnostic accuracy of PSP. Midbrain diameter in PSP (13.4 mm) was shown to be significantly lower than that of PD (18.5 mm). [48] A study indicated that the surface area of midbrain of progressive supranuclear palsy (PSP), (56 mm2) was significantly smaller than that of Parkinsonian subtype of MSA (MSA-P) (97.2 mm2), PD (103 mm2), and healthy controls (117 mm2). Some overlaps of the area measurements were observed in PSP and Parkinsonian subtype of MSA (MSA-P), but the ratio of the area of the midbrain to pons was significantly smaller in PSP when compared with Parkinsonian subtype of MSA (MSA-P). [49] On axial views, the selective atrophy of the midbrain tegmentum with relative preservation of the tectum and cerebral peduncles produces the “Mickey mouse” sign (see Fig. 6B). Sometimes, the concavity of the lateral margin of the midbrain tegmentum is referred to as the “morning glory” sign and has high specificity but rather low sensitivity for PSP. Other radiological findings of PSP include dilatation of the third ventricle, particularly the posterior portion, signal change in the periaqueductal gray matter indicative of gliosis, and atrophy of the superior cerebellar peduncle, which has a specificity of 94% and sensitivity of 74% and can aid the differentiation of PSP from MSA-P and PD. [51] “Eye of the tiger” sign with hypointensity signal change in T2, a common finding in pantothenate kinase- associated neurodegeneration (PKAN), can occasionally be observed in PSP, indicating the presence of iron deposition in the putamen.[52] Addendum A new version of this PDF file (with a new case) is uploaded in my web site every week (every Saturday and
- 16. remains available till Friday.) To download the current version follow the link "http://pdf.yassermetwally.com/case.pdf". You can also download the current version from my web site at "http://yassermetwally.com". To download the software version of the publication (crow.exe) follow the link: http://neurology.yassermetwally.com/crow.zip The case is also presented as a short case in PDF format, to download the short case follow the link: http://pdf.yassermetwally.com/short.pdf At the end of each year, all the publications are compiled on a single CD-ROM, please contact the author to know more details. Screen resolution is better set at 1024*768 pixel screen area for optimum display. Also to view a list of the previously published case records follow the following link (http://wordpress.com/tag/case-record/) or click on it if it appears as a link in your PDF reader To inspect the patient's full radiological study, click on the attachment icon (The paper clip icon in the left pane) of the acrobat reader then double click on the attached file. Click here to download the short case version of this case record in PDF format References 1. Golbe LI. Progressive Supranuclear Palsy. Curr Treat Options Neurol. Nov 2001;3(6):473-477. . 2. Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. Mar 2009;8(3):270-9. . 3. Liao K, Wagner J, Joshi A, Estrovich I, Walker MF, Strupp M, et al. Why do patients with PSP fall? Evidence for abnormal otolith responses. Neurology. Mar 4 2008;70(10):802-9. . 4. Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC. Prevalence and natural history of progressive supranuclear palsy. Neurology. Jul 1988;38(7):1031-4. . 5. Jackson JA, Jankovic J, Ford J. Progressive supranuclear palsy: clinical features and response to treatment in 16 patients. Ann Neurol. Mar 1983;13(3):273-8. . 6. Mastaglia FL, Grainger K, Kee F, et al. Progressive supranuclear palsy (the Steele-Richardson-Olszewski syndrome) clinical and electrophysiological observations in eleven cases. Proc Aust Assoc Neurol. 1973;10 (0):35-44. . 7. Maher ER, Lees AJ. The clinical features and natural history of the Steele-Richardson- Olszewski syndrome (progressive supranuclear palsy). Neurology. Jul 1986;36(7):1005-8. . 8. Kristensen MO. Progressive supranuclear palsy–20 years later. Acta Neurol Scand. Mar 1985;71(3):177-89. . 9. Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogenous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. Apr 1964;10:333-59. . 10. Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson- Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry. Jun 1996;60(6):615-20. . 11. Barclay CL, Lang AE. Dystonia in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. Apr 1997;62(4):352-6. . 12. Gibb WR, Luthert PJ, Marsden CD. Corticobasal degeneration. Brain. Oct 1989;112 ( Pt 5):1171-92. . REFERENCES
- 17. 13. Sakakibara R, Hattori T, Tojo M, et al. Micturitional disturbance in progressive supranuclear palsy. J Auton Nerv Syst. Nov 1993;45(2):101-6. . 14. Tolosa E, Espuna M, Valls J. Bladder dysfunction in PSP and other parkinsonian disorders. Mov Disord. 1997;12:272. 15. Josephs KA, Duffy JR. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol. Dec 2008;21(6):688-92. . 16. Litvan I, Mega MS, Cummings JL, Fairbanks L. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology. Nov 1996;47(5):1184-9. . 17. Litvan I, Agid Y, Jankovic J, et al. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology. Apr 1996;46(4):922-30. . 18. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. Jul 1996;47(1):1-9. . 19. Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. Nov 1994;44(11):2015-9. . 20. Golbe LI, Rubin RS, Cody RP, et al. Follow-up study of risk factors in progressive supranuclear palsy. Neurology. Jul 1996;47(1):148-54. . 21. Tetrud JW, Golbe LI, Forno LS, Farmer PM. Autopsy-proven progressive supranuclear palsy in two siblings. Neurology. Apr 1996;46(4):931-4. . 22. Kaat LD, Boon AJ, Azmani A, Kamphorst W, Breteler MM, Anar B, et al. Familial aggregation of parkinsonism in progressive supranuclear palsy. Neurology. Jul 14 2009;73(2):98-105. . 23. de Yebenes JG, Sarasa JL, Daniel SE, Lees AJ. Familial progressive supranuclear palsy. Description of a pedigree and review of the literature. Brain. Oct 1995;118 ( Pt 5):1095-103. . 24. Conrad C, Andreadis A, Trojanowski JQ, et al. Genetic evidence for the involvement of tau in progressive supranuclear palsy. Ann Neurol. Feb 1997;41(2):277-81. . 25. Borroni B, Malinverno M, Gardoni F, Alberici A, Parnetti L, Premi E, et al. Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy. Neurology. Nov 25 2008;71(22):1796-803. . 26. Drayer BP, Olanow W, Burger P, et al. Parkinson plus syndrome: diagnosis using high field MR imaging of brain iron. Radiology. May 1986;159(2):493-8. . 27. Schonfeld SM, Golbe LI, Sage JI, et al. Computed tomographic findings in progressive supranuclear palsy: correlation with clinical grade. Mov Disord. 1987;2(4):263-78. . 28. Stern MB, Braffman BH, Skolnick BE, et al. Magnetic resonance imaging in Parkinson”s disease and parkinsonian syndromes. Neurology. Nov 1989;39(11):1524-6. . 29. Savoiardo M, Girotti F, Strada L, Ciceri E. Magnetic resonance imaging in progressive supranuclear palsy and other parkinsonian disorders. J Neural Transm Suppl. 1994;42:93-110. . 30. Paviour DC, Price SL, Stevens JM, et al. Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. Feb 22 2005;64(4):675-9. . 31. Righini A, Antonini A, De Notaris R, et al. MR imaging of the superior profile of the midbrain: differential diagnosis between progressive supranuclear palsy and Parkinson disease. AJNR Am J Neuroradiol. Jun-Jul
- 18. 2004;25(6):927-32. . 32. Foster NL, Gilman S, Berent S, et al. Cerebral hypometabolism in progressive supranuclear palsy studied with positron emission tomography. Ann Neurol. Sep 1988;24(3):399-406. . 33. Foster NL, Gilman S, Berent S, et al. Progressive subcortical gliosis and progressive supranuclear palsy can have similar clinical and PET abnormalities. J Neurol Neurosurg Psychiatry. Aug 1992;55(8):707-13. . 34. Blin J, Baron JC, Dubois B, et al. Positron emission tomography study in progressive supranuclear palsy. Brain hypometabolic pattern and clinicometabolic correlations. Arch Neurol. Jul 1990;47(7):747-52. . 35. Mishina M, Ishii K, Mitani K, et al. Midbrain hypometabolism as early diagnostic sign for progressive supranuclear palsy. Acta Neurol Scand. Aug 2004;110(2):128-35. . 36. Brooks DJ, Ibanez V, Sawle GV, et al. Differing patterns of striatal 18F-dopa uptake in Parkinson”s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. Oct 1990;28(4):547-55. . 37. Arnold G, Tatsch K, Oertel WH, et al. Clinical progressive supranuclear palsy: differential diagnosis by IBZM- SPECT and MRI. J Neural Transm Suppl. 1994;42:111-8. . 38. Aldrich MS, Foster NL, White RF, et al. Sleep abnormalities in progressive supranuclear palsy. Ann Neurol. Jun 1989;25(6):577-81. . 39. Santamaria J, Iranzo A. Alteraciones del sueno en los trastornos del movimiento. Neurologia. 1997;12 (Suppl 3):35-47. 40. Gross RA, Spehlmann R, Daniels JC. Sleep disturbances in progressive supranuclear palsy. Electroencephalogr Clin Neurophysiol. Jul 1978;45(1):16-25. . 41. Laffont F, Autret A, Minz M, et al. [Polygraphic sleep recordings in 9 cases of Steele-Richardson''s disease (author''s transl)]. Rev Neurol (Paris). Feb 1979;135(2):127-41. . 42. Sixel-Döring F, Schweitzer M, Mollenhauer B, Trenkwalder C. Polysomnographic findings, video-based sleep analysis and sleep perception in progressive supranuclear palsy. Sleep Med. Apr 2009;10(4):407-15. . 43. Polo KB, Jabbari B. Botulinum toxin-A improves the rigidity of progressive supranuclear palsy. Ann Neurol. Feb 1994;35(2):237-9. . 44. Stamelou M, Reuss A, Pilatus U, Magerkurth J, Niklowitz P, Eggert KM, et al. Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial. Mov Disord. May 15 2008;23(7):942-9. . 45. Zampieri C, Di Fabio RP. Improvement of gaze control after balance and eye movement training in patients with progressive supranuclear palsy: a quasi-randomized controlled trial. Arch Phys Med Rehabil. Feb 2009;90(2):263-70. . 46. Albers DS, Augood SJ. New insights into progressive supranuclear palsy. Trends Neurosci. Jun 2001;24 (6):347-53. . 47. Hamilton SR. Neuro-ophthalmology of movement disorders. Curr Opin Ophthalmol. Dec 2000;11(6):403-7. . 48. Jankovic J, Friedman DI, Pirozzolo FJ, McCrary JA. Progressive supranuclear palsy: motor, neurobehavioral, and neuro- ophthalmic findings. Adv Neurol. 1990;53:293-304. . 49. Kuniyoshi S, Riley DE, Zee DS, et al. Distinguishing progressive supranuclear palsy from other forms of Parkinson”s disease: evaluation of new signs. Ann N Y Acad Sci. Apr 2002;956:484-6. . 50. Laffont F, Autret A, Minz M, et al. [Polygraphic study of nocturnal sleep in three degenerative diseases: ALS,
- 19. oligo-ponto-cerebellar atrophy, and progressive supranuclear palsy]. Waking Sleeping. Jan 1979;3(1):17-30. . 51. Leigh JR, Zee DS. The neurology of eye movements. 3rd ed. New York, NY:. Oxford University Press;1999:521-525. 52. Litvan I. Diagnosis and management of progressive supranuclear palsy. Semin Neurol. 2001;21(1):41-8. . 53. Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. Jan 1996;55 (1):97-105. . 54. Mark MH. Lumping and splitting the Parkinson Plus syndromes: dementia with Lewy bodies, multiple system atrophy, progressive supranuclear palsy, and cortical-basal ganglionic degeneration. Neurol Clin. Aug 2001;19(3):607-27, vi. . 55. Nath U, Ben-Shlomo Y, Thomson RG, et al. The prevalence of progressive supranuclear palsy (Steele- Richardson-Olszewski syndrome) in the UK. Brain. Jul 2001;124(Pt 7):1438-49. . 56. Osaki Y, Ben-Shlomo Y, Lees AJ, et al. Accuracy of clinical diagnosis of progressive supranuclear palsy. Mov Disord. Feb 2004;19(2):181-9. . 57. Rafal RD, Friedman JH. Limb dystonia in progressive supranuclear palsy. Neurology. Sep 1987;37(9):1546-9. . 58. Schrag A, Selai C, Davis J, et al. Health-related quality of life in patients with progressive supranuclear palsy. Mov Disord. Dec 2003;18(12):1464-9. 59. Tolosa E, Valldeoriola F. Progressive supranuclear palsy. In: Jankovic J, Tolosa E, eds. Parkinson’s Disease and Movement Disorders. 3rd ed. Baltimore, Md:. William & Wilkins;1998:221-243. 60. Warmuth-Metz M, Naumann M, Csoti I, Solymosi L. Measurement of the midbrain diameter on routine magnetic resonance imaging: a simple and accurate method of differentiating between Parkinson disease and progressive supranuclear palsy. Arch Neurol. Jul 2001;58(7):1076-9. . 61. Arima K, and others. Two brothers with frontotemporal dementia and parkinsonism with an N279K mutation of the tau gene. Neurology 2000:54:1787-1795 62. Arvin KL and others. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol 2002:52:54-61 63. Baker KB, Montgomery EB. Performance on the PD test battery by relatives of patients with progressive supranuclear palsy. Neurology 2001:56:25-30 64. Bennett and others. Direct genetic evidence for involvement of tau in progressive supranuclear palsy. Neurology 1998:51:982-985 65. Bensimon, G., A. Ludolph, et al. (2009). "Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study." Brain 132(Pt 1): 156-71. 66. Biosse V and others. Opthalmologic features of Parkinson’s disease. Neurology 2004:2:177-180 67. Boroni B and many others. Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy. Neurology 2008:71:1796-1803 68. Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976-1990. Neurology 1997:49:1284-1288 69. Caparros-Lefebvre D. Possible relation of atypical parkinsonism in the french West INdies with consumption
- 20. of tropical plants: a case-control study. Lancet 354(9175):281-286, 1999 70. Chen J and others. Statins induce angiogenecis, neurogenesis and synaptogenesis after stroke. Ann Neurol 2003:53:743-51 71. Cole DG, Growdon JH. Therapy for progressive supranuclear palsy, past and future. J.Neural Transm 1994:42:283-290 72. Cordato NJ, Halliday GM, Harding AJ, Hely MA, Morris JGL. Regional brain atrophy in progressive supranuclear palsy and Lewy body disease. 73. Cox PA, Sacks OW. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology 20002 58:956-959 74. Delacourte A and others. Vulnerable neuronal subsets in Alzheimers and Pick’s disease are distinguished by their tau isoform distribution and phosphorylation. Annals of Neurology 43(2): 193-204, 1998 75. Di Maria et al. Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann Neurol 2000:47:374-377 76. Engel PA (1996). "Treatment of progressive supranuclear palsy with amitriptyline: therapeutic and toxic effects." J Am Geriatr Soc 44(9): 1072-4. 77. Foster NL, and others. PET measures of benzodiazepine receptors in progressive supranuclear palsy. Neurology 2000, 54:1768-1773 78. Fratelli CM, Sonies BC, Chi-Fishman G, Litvin I. Effects of physostigmine on swallowing and oral motor functions in patients with progressive supranuclar palsy: A pilot study. Dysphagia 14(3):165-168, 1999. Comment: this medication did not help. 79. Goetz CG, Leurgans S, Lang AE and Litvan I (2003). "Progression of gait, speech and swallowing deficits in progressive supranuclear palsy." Neurology 60(6): 917-22. 80. Houlden H and others. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 2001:56:1702-6. 81. Glika J, Bogousslavsky J. Presymptomatic hypertension is a major feature in the diagnosis of progressive supranuclear palsy. Arch Neurl 54(9):1104-1108, 1997. Comment: hypertension is so common that this observation, even if true, is of limited significance. 82. Golbe LI. New survey finds PSP five-fold more common than thought. PSP advocate, 2000 Comment: this study does not really document that PSP is 5 fold more common, this figure is nearly the same as was found in the study of Bower, published in 1997 (TCH). This study actually simply shows that an older study done in 1987 was incorrect. 83. Golbe LI. PSP research update. (PSP Advocate, 3rd issue, 2004) The 8th international congress on Parkinson’s disease and Movement Disorders, June 2004. 84. Higgins JJ, Litvan I, Pho LT, Li W, Nee LE. Progresive supranuclear palsy is in linkage disequilibrium with the tau and not the alpha-synuclein gene. Neurology 1998:50:270-273 85. Higgins JJ, Litvan I, Nee LE, Loveless BS. A lack of the R406W tau mutation in progressive supranuclear palsy and corticobasal degeneration. Neurology 1999:52:404-406 86. Higgins JL, Adler RL, Loveless JM. Mutational analysis of the tau gene in progressive supranuclear palsy. Neurology 1999:53:1421-1424 87. Jellinger KA, Bancher C. Neuropathology in: Progressive Supranuclear Palsy: Clinical and Research
- 21. Approaches, Litvin I, Agid Y (eds) New York:Oxford 1992, 44-88 88. Katzenschlager R and others. Fourteen-year final report of the randomized PDRG-UK trial comparing three initial treatments in PD. Neurology 2008:71:474-480 89. Kris J, Gowing G, Julien JP. Efficient three-drug cocktail for disease induced by mutant superoxide dismutates. Ann Neurol 2003:53:429-436 90. Kuniyoshi, S., D. E. Riley, D. S. Zee, S. G. Reich, C. Whitney and R. J. Leigh (2002). "Distinguishing progressive supranuclear palsy from other forms of Parkinson’s disease: evaluation of new signs." Ann N Y Acad Sci 956: 484-6. 91. Liao K and others. Why do patients with PSP fall ? Evidence for abnormal otolith responses. Neurology 2008; 70:802-9 92. Litvan I, Sastry N, Sonies B. Characterizing swallowing abnormalities in progressive supranuclear palsy. Neurology 1997:48:1654-1662 93. Litvan I, Campbell G, Mangone CA, Verny M, McKee A, Chaudhuri K, Jellinger K, Pearce RKB, D’Olhaberriague LD. Which clinical features differentiate progressive supranuclear palsy (Steele- Richardson-Olszewski syndrome) from related disorders ? A clinicopathological study. Brain (1997), 120, 65- 74 94. Litvan, I., M. Phipps, et al. (2001). "Randomized placebo-controlled trial of donepezil in patients with progressive supranuclear palsy." Neurology 57(3): 467-73. 95. Martin and others. Association of single-nucleotide polymorphisms of the Tau gene with late-onset parkinson disease. JAMA 2001:2245-2250 96. Maurage CA and others. Similar brain tau pathology in DM2/PROMM and DM1/Steinert Disease. Neurology 2005:65:1636-1638 97. Mokri B, and others. Syndrome resembling PSP after surgical repair of ascending aorta dissection or aneurysm. Neurology 2004, 62:971-73 98. Moro DA, Bentivoglio AR. Zolpidem in progressive supranuclar palsy. NEJM 341(7):543-544, 1999. Comment: benzodiazepines are effective in other central ataxias, and might also be effective in PSP 99. Murrell JR, Koller D and others. Familial multi-system tauopathy with presenile dementia is localized to chromosome 17. Am J. Hum. Gen 61(5):1131-8, 1997 100. Nagahima Y and others. Movement Disorders 12(5) 691-696, 1997. Comment: This study suggests that PET scans can differentiated CGBD from PSP. Unfortunately, PET scans are so expensive that this information is of minimal clinical value. 101. Nath U, Ben-Shlomo Y, Thomson RG, Lees AJ and Burn DJ (2003). "Clinical features and natural history of progressive supranuclear palsy: A clinical cohort study." Neurology 60(6): 910-6. 102. Oba H and many others. New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology 2005: 64: 2050-2055 103. Olanow CW, Fahn S, Langston JW, Godbold J. Selegiline and Mortaility in Parkinson’s Disease. Ann Neurol 40,6, Dec 1996. 104. Otto M and others. Efficacy of flupirtine on cognitive function in patients with CJD: A double-blind study. Neurology 2004:62:714-718 105. Oskarsson B and others. Stiff eyes in stiff-person syndrome. Neurology 71, 2008, 378-379
- 22. 106. Pavior and others. Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 2005:64:675-679 107. Reed LA, Wszolek ZK, Hutton M. Phenotypic correlations in FDTP-17. Neurobiology of Aging 22(2001) 89- 107. 108. Rojo et al. Clinical genetics of familial progressive supranuclear palsy. Brain 122(7) 1233-1245, 1999 109. Santacruz P, Uttl B, Litvan I, Grafman J. Progresive supranuclear palsy. A survey of the disease course. Neurology 1998:50:1637-1647 110. Schrag A, Ben-Shlomo Y. Quinn NP. Prevalence of progressive supranuclaear palsy and multiple system atrophy: a cross-sectional study. Lancet 354(9192):1771-1775, 1999. 111. Schiffmann et al. Prospective study of neurological responses &ldots; Ann Neurol 1997, 42:613-621 (Gaucher’s disease). 112. Schmidt, C., B. Herting, et al. (2009). "Valsalva manoeuvre in patients with different Parkinsonian disorders." J Neural Transm. 113. Schneider, A. and E. Mandelkow (2008). "Tau-based treatment strategies in neurodegenerative diseases." Neurotherapeutics 5(3): 443-57. 114. Sergeant N, and others. Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively "exon 10" isoforms. J. Neurochem 1999;72:1243-49 115. Seppi K, Schocke MF, Esterhammer R, Kremser C, Brenneis C, Mueller J, Boesch S, Jaschke W, Poewe W and Wenning GK (2003). "Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the parkinson variant of multiple system atrophy." Neurology 60(6): 922-7. 116. Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative disease. Trends Neurosci 1998:21:428-433 117. Spillantini MG, Goedert M. Tau and Parkinson Disease. JAMA 2001, 286, 2324-2324 118. Stamelou, M., A. Reuss, et al. (2008). "Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial." Mov Disord 23(7): 942-9. 119. Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. Arch Neurol 1964:10:333-359. 120. Suteerawattananon M, MacNeill B, Protas EJ. Supported treadmill training for gait and balanace in a patient with progressive supranuclar palsy. Phys Ther 2002:82:485-495 121. Tanagawa A, Komiyama A, Hasegawa O. Truncal muscle tonus in progressive supranuclear palsy. Journal of Neurol, Neurosurg and Psych, 64(2):190-196, 1998. Comment: This article suggests that the neck is stiff but the trunk is not stiff. This study needs to be repeated and confirmed. 122. Tsuboi Y, Ahlskog JE and others. Lewy bodies are not increased in progressive supranuclear palsy compared with normal controls. Neurology 2001:57:1675-78 123. Yamamoto, M. and A. H. Schapira (2008). "Dopamine agonists in Parkinson’s disease." Expert Rev Neurother 8(4): 671-7. 124. Weiner WJ, Minager A, Shulman LM. Pramipexole in progressive supranuclear palsy. Neurology 1999:52:873-874. Comment: this study reported no effect. 125. Wzzolek Z, Uitti RJ, Hutton M. A mutation in the microtubule-associated protein tau in pallido-nigro-luysian degeneration. Neurology 54, 2028, 2000
- 23. 126. Zampieri, C. and R. P. Di Fabio (2008). "Balance and eye movement training to improve gait in people with progressive supranuclear palsy: quasi-randomized clinical trial." Phys Ther 88(12): 1460-73. 127. The idiopathic parkinson disease: A magnetic resonance imaging study with correlation of the clinical picture and the pattern of levodopa responsiveness. Metwally, MYM (2000): Ain Shams medical journal, VOL 51, No 1,2,3, pp 181-198 128. Metwally, MYM: Textbook of neuroimaging, A CD-ROM publication, (Metwally, MYM editor) WEB-CD agency for electronic publication, version 9.4a October 2009
