Micro chapter 31 biofilms - architects of disease
•
8 likes•3,168 views
Biofilm powerpoint
Report
Share
Report
Share
Download to read offline
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Formation Structure and Internal Functions of Microbial mats And Biofilms

Formation Structure and Internal Functions of Microbial mats And Biofilms
bioplastics by microorganisms Polyhydroxyalkanoates And Polyhydroxybutyrate

bioplastics by microorganisms Polyhydroxyalkanoates And Polyhydroxybutyrate
Identification of gram positive and gram negative bacteria

Identification of gram positive and gram negative bacteria
Viewers also liked
Viewers also liked (8)
Cleaning and disinfection of p atient care equipment

Cleaning and disinfection of p atient care equipment
Hospital Environmental Cleaning & Disinfection, Procedures & Practices

Hospital Environmental Cleaning & Disinfection, Procedures & Practices
Similar to Micro chapter 31 biofilms - architects of disease
Similar to Micro chapter 31 biofilms - architects of disease (20)
Recently uploaded
Ahmedabad Call Girls Book Now 9630942363 Top Class Ahmedabad Escort Service Available
9630942363 Ahmedabad Escort Service Ahmedabad Call Girls Ahmedabad Escorts Service Ahmedabad Call Girl Russian 9630942363 Russian Ahmedabad Escort Service Vip Ahmedabad Escort Service Ahmedabad Call Girls Housewife Model College Girls Aunty Bhabhi Ahmedabad 9630942363 Genuine Ahmedabad Call Girl Escort ServiceAhmedabad Call Girls Book Now 9630942363 Top Class Ahmedabad Escort Service A...

Ahmedabad Call Girls Book Now 9630942363 Top Class Ahmedabad Escort Service A...GENUINE ESCORT AGENCY
Recently uploaded (20)
Call Girls Bangalore - 450+ Call Girl Cash Payment 💯Call Us 🔝 6378878445 🔝 💃 ...

Call Girls Bangalore - 450+ Call Girl Cash Payment 💯Call Us 🔝 6378878445 🔝 💃 ...
ANATOMY AND PHYSIOLOGY OF REPRODUCTIVE SYSTEM.pptx

ANATOMY AND PHYSIOLOGY OF REPRODUCTIVE SYSTEM.pptx
Chandigarh Call Girls Service ❤️🍑 9809698092 👄🫦Independent Escort Service Cha...

Chandigarh Call Girls Service ❤️🍑 9809698092 👄🫦Independent Escort Service Cha...
Dehradun Call Girl Service ❤️🍑 8854095900 👄🫦Independent Escort Service Dehradun

Dehradun Call Girl Service ❤️🍑 8854095900 👄🫦Independent Escort Service Dehradun
Gorgeous Call Girls Dehradun {8854095900} ❤️VVIP ROCKY Call Girls in Dehradun...

Gorgeous Call Girls Dehradun {8854095900} ❤️VVIP ROCKY Call Girls in Dehradun...
Ahmedabad Call Girls Book Now 9630942363 Top Class Ahmedabad Escort Service A...

Ahmedabad Call Girls Book Now 9630942363 Top Class Ahmedabad Escort Service A...
7 steps How to prevent Thalassemia : Dr Sharda Jain & Vandana Gupta

7 steps How to prevent Thalassemia : Dr Sharda Jain & Vandana Gupta
Whitefield { Call Girl in Bangalore ₹7.5k Pick Up & Drop With Cash Payment 63...

Whitefield { Call Girl in Bangalore ₹7.5k Pick Up & Drop With Cash Payment 63...
Premium Call Girls Nagpur {9xx000xx09} ❤️VVIP POOJA Call Girls in Nagpur Maha...

Premium Call Girls Nagpur {9xx000xx09} ❤️VVIP POOJA Call Girls in Nagpur Maha...
Pune Call Girl Service 📞9xx000xx09📞Just Call Divya📲 Call Girl In Pune No💰Adva...

Pune Call Girl Service 📞9xx000xx09📞Just Call Divya📲 Call Girl In Pune No💰Adva...
🚺LEELA JOSHI WhatsApp Number +91-9930245274 ✔ Unsatisfied Bhabhi Call Girls T...

🚺LEELA JOSHI WhatsApp Number +91-9930245274 ✔ Unsatisfied Bhabhi Call Girls T...
👉 Chennai Sexy Aunty’s WhatsApp Number 👉📞 7427069034 👉📞 Just📲 Call Ruhi Colle...

👉 Chennai Sexy Aunty’s WhatsApp Number 👉📞 7427069034 👉📞 Just📲 Call Ruhi Colle...
Race Course Road } Book Call Girls in Bangalore | Whatsapp No 6378878445 VIP ...

Race Course Road } Book Call Girls in Bangalore | Whatsapp No 6378878445 VIP ...
Nagpur Call Girl Service 📞9xx000xx09📞Just Call Divya📲 Call Girl In Nagpur No💰...

Nagpur Call Girl Service 📞9xx000xx09📞Just Call Divya📲 Call Girl In Nagpur No💰...
Bhawanipatna Call Girls 📞9332606886 Call Girls in Bhawanipatna Escorts servic...

Bhawanipatna Call Girls 📞9332606886 Call Girls in Bhawanipatna Escorts servic...
👉Chandigarh Call Girl Service📲Niamh 8868886958 📲Book 24hours Now📲👉Sexy Call G...

👉Chandigarh Call Girl Service📲Niamh 8868886958 📲Book 24hours Now📲👉Sexy Call G...
👉 Amritsar Call Girls 👉📞 8725944379 👉📞 Just📲 Call Ruhi Call Girl Near Me Amri...

👉 Amritsar Call Girls 👉📞 8725944379 👉📞 Just📲 Call Ruhi Call Girl Near Me Amri...
💰Call Girl In Bangalore☎️63788-78445💰 Call Girl service in Bangalore☎️Bangalo...

💰Call Girl In Bangalore☎️63788-78445💰 Call Girl service in Bangalore☎️Bangalo...
Micro chapter 31 biofilms - architects of disease
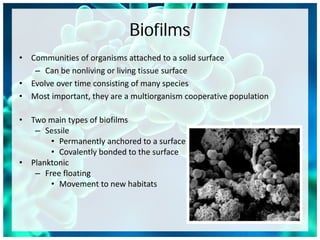
- 1. Biofilms • Communities of organisms attached to a solid surface – Can be nonliving or living tissue surface • Evolve over time consisting of many species • Most important, they are a multiorganism cooperative population • Two main types of biofilms – Sessile • Permanently anchored to a surface • Covalently bonded to the surface • Planktonic – Free floating • Movement to new habitats
- 2. Biofilms (Cont’d) • Examples of biofilms – Water pipes – Ventilator system of airplanes or convention centers – Wine casks causing spoilage – Serious lung infections of CF patients • Problems with susceptibility
- 3. Natural Biofilm on a Metal Surface
- 4. Environmental and Cultural Factors That Affect Biofilm Development
- 5. Biofilms: Community of Cells • Most important characteristics – Attachment efficiency – Nutritional resources – Substrata – Environment shear stress or force
- 6. Variables Important in Cell Attachment and Biofilm Formation
- 7. Stage I – Development I Stage II – Development II • Reversible binding to surface • Increased attachment via fimbriae and pili • Irreversible binding and aggregate formation • Decreased motility • Exopolysaccharide (EPS) trap nutrients and planktonic bacteria Stage III – Maturation I Stage IV – Maturation II • Colony thickness of greater than 10 um thick • Colony thickness of greater than 100 um thick • Some bacteria detach but are trapped in the film Stage V Stage 0 • Breaking off of bacteria leads to start of new biofilms • Planktonic state
- 8. Stages in Biofilm Formation (Cont’d) • Active growing cells • Persister cells – Cells in a dormancy-like state • Importance – Cells not actively growing may not be affected by drugs » Cell wall inhibitors » Ribosome inhibitors • Communication between bacteria – Quorum sensing (QS) • Pheromones – Gram positive – low-molecular-weight homoserines – Gram negative – peptides and proteins
- 9. Architecture of Biofilms • Outer layer – Most dynamic and metabolically active cells • Intermediate layer – Still active but less so – Genetic reservoir for genes involving nutrient utilization and drug resistance • Inner surface layer – Persister cells • Dynamic system – Defends itself as a group • Freely exchanging traits and retaining resistance
- 11. Biofilms as a Defense Mechanism • When culturing organisms – Catheter tips, artificial joints, etc. – Isolation of individual organisms can be hard to culture • Sessile – Isolated colonies may not reflect the colonies permanently attached to the plastic surface • Planktonic – Isolated colonies may not contain antibiotic resistance, but other colonies in the group may contain resistance • Look susceptible in a dish but not in the patient – Treatment failure
- 12. Biofilms as a Defense Mechanism (Cont’d) • Protect against pH changes • Interference with immune function – Prevent phagocytosis – Prevent antigen exposure to antibodies • Sticky EPS glues biofilm together; stops clearance • Organization of biofilm – Slow-growing organisms attached to surface show increase resistance to antibiotics
- 13. Biofilms as a Defense Mechanism (Cont’d) • Gene transfer – Transformation – Conjugation • Greater genetic potential as a group than alone – Eventually the virulence factors cluster, causing a worsening of disease
- 14. Diseases Associated with Biofilms • Primarily indwelling medical devices – Examples include • Artificial heart valves • Prostheses • Catheters • Can be tissue and vessels as well – Some disease as it progresses from acute to chronic diseases
- 15. Dental Biofilms • Plaque – Caries (cavities) – Periodontal disease • Dental cleaning removes plaque – Biofilm develops again • Acquired pellicle – Organisms produce glycans to produce slime layer • Sugars – Broken down to acids that damage teeth
- 16. Laboratory Consequences Associated with Biofilms • Cultures – Require growth to get colonies • Problem is colonies won’t grow under normal conditions • False negatives – Improper sample collection • Swabs or culturing outer surface of equipment • Aggregates of organisms – Single colonies can represent up to 100,000 bacteria of mixed origin • Thus amounts of each organism are greatly underestimated or not considered significant • Antibiotic susceptibility – Single isolates that are members of a biofilm may not represent the genetic potential or resistance of a community
- 17. Detection of Biofilms • PCR with pathogen-specific probes • Confocal laser scanning microscopic imaging – CLSM
- 18. Potential Interventions • Establishing biofilms in 96 well plates – Minimal biofilm elimination concentration (MBEC) • Help select successful concentrations of drugs and appropriate concentration • Treatment outcomes – Prevent metastasis – Reduce bioburden – Prevent attachment • Other treatments – Sonication to disrupt biofilm – Toxic compounds (silver)