Report
Share
More Related Content
What's hot (20)
Major extracellular and intracellular electrolytes

Major extracellular and intracellular electrolytes
Viewers also liked
Play as Product: How Play and Playfulness can help us build better Products a...

Play as Product: How Play and Playfulness can help us build better Products a...Rosemary Elizabeth King
Viewers also liked (20)
Second Marshall Cavendish Singapore Mathematics Global Forum 2011

Second Marshall Cavendish Singapore Mathematics Global Forum 2011
Play as Product: How Play and Playfulness can help us build better Products a...

Play as Product: How Play and Playfulness can help us build better Products a...
SEO Content Contributors by Andrea H. Berberich @webpresenceopti

SEO Content Contributors by Andrea H. Berberich @webpresenceopti
Азбука схемы принятия решения - как продавать большим компаниям

Азбука схемы принятия решения - как продавать большим компаниям
HTML5: Building the Next Generation of Web Applications

HTML5: Building the Next Generation of Web Applications
Windows Azure: Verbinden, erweitern, integrieren Sie ihr Firmennetzwerk in di...

Windows Azure: Verbinden, erweitern, integrieren Sie ihr Firmennetzwerk in di...
Similar to Chemistry
Study Material-Organic Chemistry - Some Basic Principles and Techniques Class...

Study Material-Organic Chemistry - Some Basic Principles and Techniques Class...Vivekanand Anglo Vedic Academy
Similar to Chemistry (20)
M.SC SEM2.ADITI BAJPAI.PAPER3water purification system.Z_22_40.docx.pptx

M.SC SEM2.ADITI BAJPAI.PAPER3water purification system.Z_22_40.docx.pptx
Plan an ETP with detail process discussion following the instructions

Plan an ETP with detail process discussion following the instructions
Activated sludge process in wastewater treatment plant

Activated sludge process in wastewater treatment plant
Study Material-Organic Chemistry - Some Basic Principles and Techniques Class...

Study Material-Organic Chemistry - Some Basic Principles and Techniques Class...
Recently uploaded
Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...Association for Project Management
Mehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
Recently uploaded (20)
Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
On National Teacher Day, meet the 2024-25 Kenan Fellows

On National Teacher Day, meet the 2024-25 Kenan Fellows
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
Vishram Singh - Textbook of Anatomy Upper Limb and Thorax.. Volume 1 (1).pdf

Vishram Singh - Textbook of Anatomy Upper Limb and Thorax.. Volume 1 (1).pdf
Salient Features of India constitution especially power and functions

Salient Features of India constitution especially power and functions
Mixin Classes in Odoo 17 How to Extend Models Using Mixin Classes

Mixin Classes in Odoo 17 How to Extend Models Using Mixin Classes
HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx

HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx
UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf

UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf
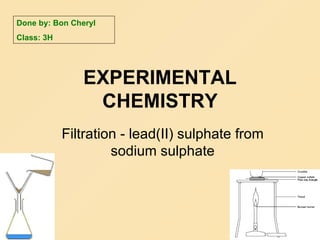
Chemistry
- 1. EXPERIMENTAL CHEMISTRY Filtration - lead(II) sulphate from sodium sulphate Done by: Bon Cheryl Class: 3H
- 3. Procedure Step 1: Prepare the lead(II) sulphate. Pour 30 cm3 of sodium sulphate into a beaker containing 50 cm3 of lead(II) nitrate solution. Stir the reaction mixture with a glass rod. Aqueous sodium sulphate Aqueous lead(II) nitrate Glass rod
- 4. Procedure Step 2: Fold the filter paper. Fold a piece of filter paper as shown. Place it in a filter funnel. Moisten it with a little distilled water.
- 5. Procedure Step 3: Filter the mixture. Pour the reaction mixture into the filter funnel that is lined with filter paper. Collect the filtrate that passes through the filter paper in a conical flask. filtrate filter paper residue reaction mixture
- 6. Procedure Step 4: Collect the residue The residue is lead(II) sulphate. Wash the residue with distilled water. Then dry the residue on a piece of filter paper. Distilled water Dry the lead(II) sulphate
- 9. Animation on filtration http://www.saskschools.ca/curr_content/science10/unita/redon17.html