Lecture 8.4b- Polar Molecules
•Download as PPT, PDF•
3 likes•3,450 views
Section 8.4 lecture (part B) for Honors & Prep Chemistry
Report
Share
Report
Share
Recommended
Polar and non polar compounds and dipole moment - PPT

Polar and non polar compounds and dipole moment - PPTSri Ramakrishna Mission Vidyalaya College of Arts and Science,Coimbatore-20.
Recommended
More Related Content
What's hot
What's hot (20)
Viewers also liked
Viewers also liked (20)
Travis County Transportation Plan: Creating Tomorrow's Choice's Today

Travis County Transportation Plan: Creating Tomorrow's Choice's Today
IB Chemistry on Polarity, Hydrogen Bonding and Van Der Waals forces

IB Chemistry on Polarity, Hydrogen Bonding and Van Der Waals forces
Similar to Lecture 8.4b- Polar Molecules
Similar to Lecture 8.4b- Polar Molecules (20)
More from Mary Beth Smith
More from Mary Beth Smith (20)
Chapter 3 and 5 lecture- Ecology & Population Growth

Chapter 3 and 5 lecture- Ecology & Population Growth
Biotechnology Chapter Five Lecture- Proteins (part b)

Biotechnology Chapter Five Lecture- Proteins (part b)
Biotechnology Chapter Five Lecture- Proteins (part a)

Biotechnology Chapter Five Lecture- Proteins (part a)
Recently uploaded
Recently uploaded (20)
DEV meet-up UiPath Document Understanding May 7 2024 Amsterdam

DEV meet-up UiPath Document Understanding May 7 2024 Amsterdam
Apidays New York 2024 - The value of a flexible API Management solution for O...

Apidays New York 2024 - The value of a flexible API Management solution for O...
Spring Boot vs Quarkus the ultimate battle - DevoxxUK

Spring Boot vs Quarkus the ultimate battle - DevoxxUK
Apidays New York 2024 - The Good, the Bad and the Governed by David O'Neill, ...

Apidays New York 2024 - The Good, the Bad and the Governed by David O'Neill, ...
Emergent Methods: Multi-lingual narrative tracking in the news - real-time ex...

Emergent Methods: Multi-lingual narrative tracking in the news - real-time ex...
Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood

Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood
AWS Community Day CPH - Three problems of Terraform

AWS Community Day CPH - Three problems of Terraform
Connector Corner: Accelerate revenue generation using UiPath API-centric busi...

Connector Corner: Accelerate revenue generation using UiPath API-centric busi...
Strategize a Smooth Tenant-to-tenant Migration and Copilot Takeoff

Strategize a Smooth Tenant-to-tenant Migration and Copilot Takeoff
EMPOWERMENT TECHNOLOGY GRADE 11 QUARTER 2 REVIEWER

EMPOWERMENT TECHNOLOGY GRADE 11 QUARTER 2 REVIEWER
Rising Above_ Dubai Floods and the Fortitude of Dubai International Airport.pdf

Rising Above_ Dubai Floods and the Fortitude of Dubai International Airport.pdf
Navigating the Deluge_ Dubai Floods and the Resilience of Dubai International...

Navigating the Deluge_ Dubai Floods and the Resilience of Dubai International...
"I see eyes in my soup": How Delivery Hero implemented the safety system for ...

"I see eyes in my soup": How Delivery Hero implemented the safety system for ...
Lecture 8.4b- Polar Molecules
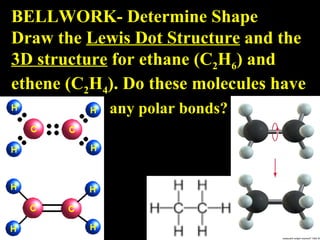
- 1. BELLWORK- Determine Shape Draw the Lewis Dot Structure and the 3D structure for ethane (C 2 H 6 ) and ethene (C 2 H 4 ). Do these molecules have any polar bonds?
- 2. In review Equal sharing of electrons = covalent LOW electronegativity difference Unequal sharing of electrons = polar MEDIUM electronegativity difference Transfer of electrons = ionic HIGH electronegativity difference
- 3. 2 bonding domains Three bonding domains Four bonding domains Three bonding and one lone pair Two bonding and two lone pairs
- 4. Bond polarity and molecule shape determine if a molecule is polar Bond polarity --- When a bond has a partial negative charge on one atom and a partial positive charge on the other atom. Molecule shape--- the arrangement of atoms in three dimensions (3-D)
- 5. A polar molecule has polar bonds and asymmetry
- 6. A polar molecule has polar bonds and asymmetry Polar bonds Non-polar molecule Symmetry- all sides are the same δ - δ - δ - δ - δ +
- 7. A polar molecule has polar bonds and asymmetry Polar bonds Non-polar molecule Symmetry- all sides are the same Polar bonds Polar molecule Asymmetry- has different sides δ - δ - δ - δ - δ - δ + δ + negative side Positive side
- 8. If the electrons are not distributed equally, the molecule is polar. The molecule has a negative end and a positive end.
- 9. A polar molecule has a partially positive side and a partially negative side. The arrow labels the molecular polarity. It shows that electrons are mostly by the oxygen atom O H H
- 10. Polar molecules are affected by electric fields
- 11. Polar molecules have two poles; one is partially positive and one is slightly negative. Positive end Negative end N H H H
- 12. The positive pole ( + ) is attracted to negative ions and the negative poles( - ) in other polar molecules. POLAR MOLECULES INTERACT!!
- 13. + POLAR MOLECULES INTERACT!! The negative pole ( - ) is attracted to positive ions and the positive poles( + ) in other polar molecules.
- 14. Water is a molecule that has two polar O-H bonds.
- 15. The electrons are not distributed evenly, so the water molecule is polar. The negative pole is at the oxygen. O is more electronegative than H, so electrons are pulled toward O. Also, there are two lone pairs around oxygen. negative end positive end
- 16. Na + (aq) A dissolved sodium ion
- 17. Water is very good at dissolving salts because it can surround anions & cations.
- 18. Practice- Using last night’s homework assignment, label the molecular polarity on the four pictures H F