Recommended
Recommended
More Related Content
More from wja10255
More from wja10255 (20)
Unit 6 ch 14 s3 land management & conservation

Unit 6 ch 14 s3 land management & conservation
Unit 4 ch 17 s1 energy resources & fossil fuels

Unit 4 ch 17 s1 energy resources & fossil fuels
Unit 3 a ch 8 s2 how species interact with each other

Unit 3 a ch 8 s2 how species interact with each other
Unit 2 a ch 4 s1 ecosystems- everything is connected

Unit 2 a ch 4 s1 ecosystems- everything is connected
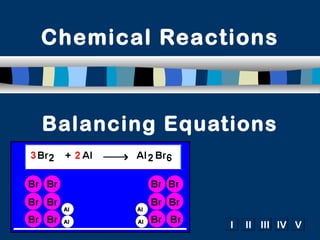
Balancing equations
- 1. Chemical Reactions Balancing Equations I II III IV V
- 2. A. Balancing Steps 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Add coefficients to make #s equal. Coefficient × subscript = # of atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!!
- 3. B. Helpful Tips s Balance one element at a time. s Update ALL atom counts after adding a coefficient. s If an element appears more than once per side, balance it last. s Balance polyatomic ions as single units. • “1 SO4” instead of “1 S” and “4 O”
- 4. C. Balancing Example Aluminum and copper(II) chloride react to form copper and aluminum chloride. 2 Al + 3 CuCl2 → 3 Cu + 2 AlCl3 ⁄ 21 Al ⁄ 1 2 ⁄ 31 Cu ⁄ 1 3 ⁄ 62 Cl ⁄ 3 6