Project Example 3
•Download as PPTX, PDF•
0 likes•225 views
The document outlines strategies for a manufacturer to encourage payers to consider the broader costs of disease rather than just the cost of medication. It recommends conducting longer randomized controlled trials to demonstrate the safety of Product X as maintenance therapy and using studies to support its use in patients with comorbidities like diabetes and osteoporosis. It also suggests creating models to show how Product X could reduce other medical costs like hospitalization and presenting cost comparisons to other treatment alternatives.
Report
Share
Report
Share
Recommended
0305 Spurrier - Ryll - Astratinei - Early access to new therapies spurrier - ...

0305 Spurrier - Ryll - Astratinei - Early access to new therapies spurrier - ...Workgroup of European Cancer Patient Advocacy Networks
More Related Content
What's hot
0305 Spurrier - Ryll - Astratinei - Early access to new therapies spurrier - ...

0305 Spurrier - Ryll - Astratinei - Early access to new therapies spurrier - ...Workgroup of European Cancer Patient Advocacy Networks
What's hot (11)
White_Paper_Synthesis of Adaptive Designs in Clinical Trials_May2016.PDF

White_Paper_Synthesis of Adaptive Designs in Clinical Trials_May2016.PDF
0305 Spurrier - Ryll - Astratinei - Early access to new therapies spurrier - ...

0305 Spurrier - Ryll - Astratinei - Early access to new therapies spurrier - ...
Evidence live 2015 -hierarchical levels of evidence based medicine are incor...

Evidence live 2015 -hierarchical levels of evidence based medicine are incor...
Evaluating Highly Specialised Technologies -- An Economist's View

Evaluating Highly Specialised Technologies -- An Economist's View
Viewers also liked
Viewers also liked (11)
PERUMIN 31: Análisis de las Bolsa de Valores de Toronto y Lima

PERUMIN 31: Análisis de las Bolsa de Valores de Toronto y Lima
Similar to Project Example 3
Ensuring Values-based Assessments for Innovative Therapies: Brent Fraser (CADTH)

Ensuring Values-based Assessments for Innovative Therapies: Brent Fraser (CADTH)Canadian Organization for Rare Disorders
Canada’s Orphan Drug Regulatory Framework & panCanadian Access to Rare Diseas...

Canada’s Orphan Drug Regulatory Framework & panCanadian Access to Rare Diseas...Canadian Organization for Rare Disorders
Canada’s Orphan Drug Regulatory Framework & panCanadian Access to Rare Diseas...

Canada’s Orphan Drug Regulatory Framework & panCanadian Access to Rare Diseas...Canadian Organization for Rare Disorders
Similar to Project Example 3 (20)
AHP Medicines Project - Improving quality and productivity

AHP Medicines Project - Improving quality and productivity
Defining Innovation in Medicines: How to Transform Innovation into Value

Defining Innovation in Medicines: How to Transform Innovation into Value
What’s Next in US Payor Communications: The Impact of FDA's Proposed Guidance...

What’s Next in US Payor Communications: The Impact of FDA's Proposed Guidance...
Ensuring Values-based Assessments for Innovative Therapies: Brent Fraser (CADTH)

Ensuring Values-based Assessments for Innovative Therapies: Brent Fraser (CADTH)
Apr 13 improving methods and processes codependent techs mg

Apr 13 improving methods and processes codependent techs mg
Balancing post-market monitoring with pre-market requirements

Balancing post-market monitoring with pre-market requirements
How to promote the prescription of evidence-based non-pharmacological treatme...

How to promote the prescription of evidence-based non-pharmacological treatme...
Medicines optimisation, pop up uni, 9am, 3 september 2015

Medicines optimisation, pop up uni, 9am, 3 september 2015
Improving Methods and Processes for Assessing Codependent Technologies

Improving Methods and Processes for Assessing Codependent Technologies
Canada’s Orphan Drug Regulatory Framework & panCanadian Access to Rare Diseas...

Canada’s Orphan Drug Regulatory Framework & panCanadian Access to Rare Diseas...
Canada’s Orphan Drug Regulatory Framework & panCanadian Access to Rare Diseas...

Canada’s Orphan Drug Regulatory Framework & panCanadian Access to Rare Diseas...
Project Example 3
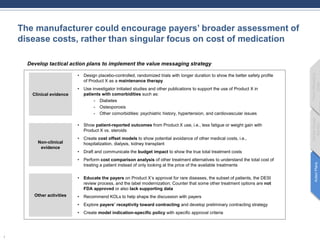
- 1. The manufacturer could encourage payers’ broader assessment of disease costs, rather than singular focus on cost of medication 1 Develop tactical action plans to implement the value messaging strategy Clinical evidence • Design placebo-controlled, randomized trials with longer duration to show the better safety profile of Product X as a maintenance therapy • Use investigator initiated studies and other publications to support the use of Product X in patients with comorbidities such as: - Diabetes - Osteoporosis - Other comorbidities: psychiatric history, hypertension, and cardiovascular issues Non-clinical evidence Other activities • Show patient-reported outcomes from Product X use, i.e., less fatigue or weight gain with Product X vs. steroids • Create cost offset models to show potential avoidance of other medical costs, i.e., hospitalization, dialysis, kidney transplant • Draft and communicate the budget impact to show the true total treatment costs • Perform cost comparison analysis of other treatment alternatives to understand the total cost of treating a patient instead of only looking at the price of the available treatments • Educate the payers on Product X’s approval for rare diseases, the subset of patients, the DESI review process, and the label modernization; Counter that some other treatment options are not FDA approved or also lack supporting data • Recommend KOLs to help shape the discussion with payers • Explore payers’ receptivity toward contracting and develop preliminary contracting strategy • Create model indication-specific policy with specific approval criteria ActionPlans MarketAccess Barriers PayerMessaging Goals
