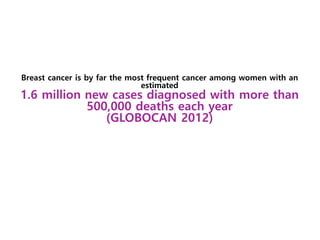
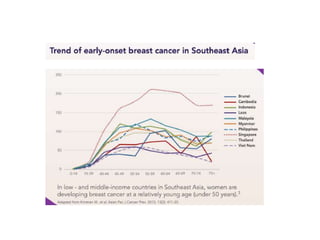
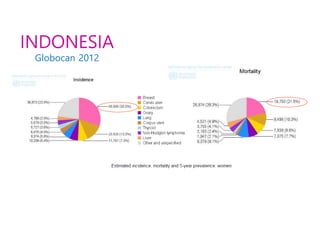
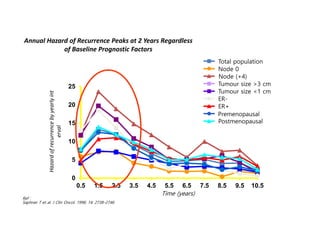
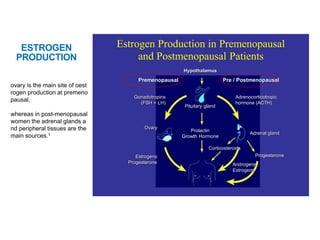
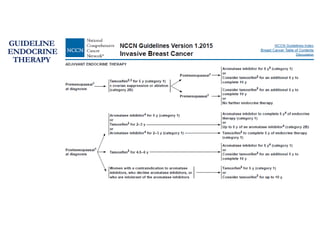
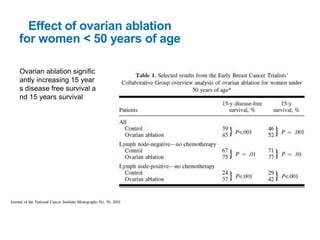
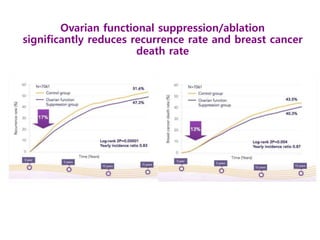
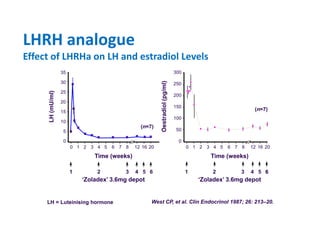
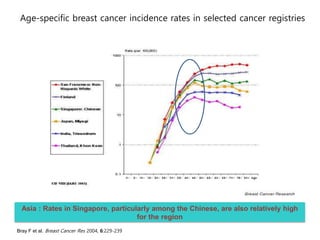
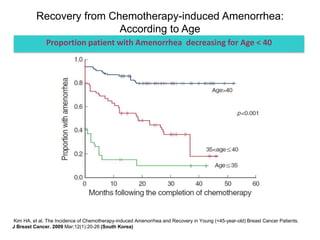
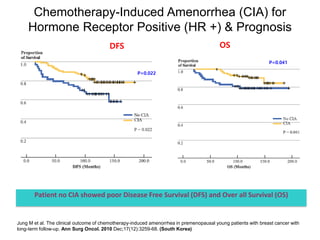
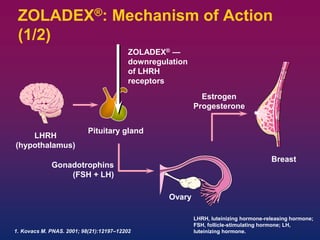
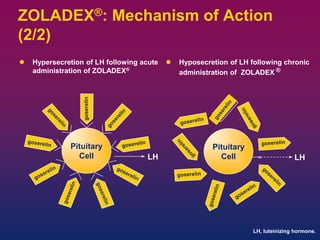
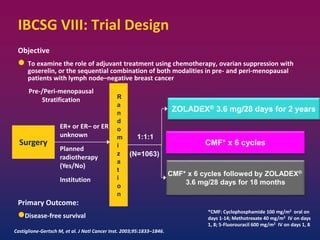
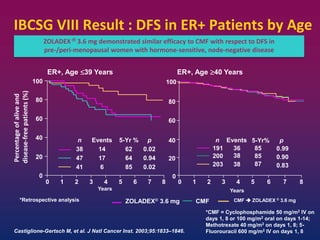
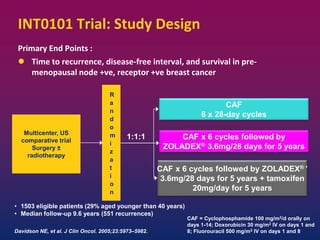
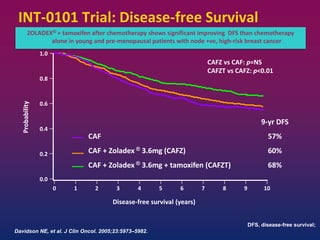
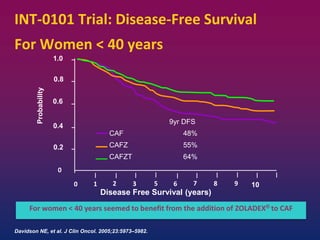
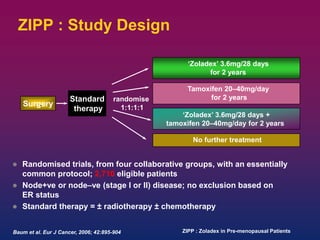
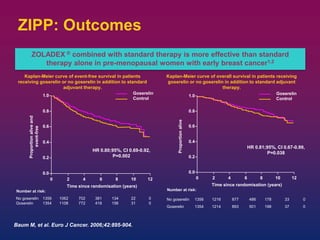
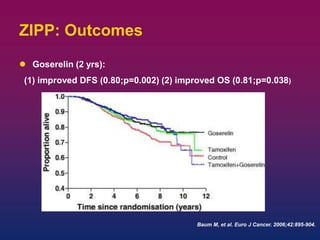
Breast cancer is the most common cancer in women worldwide, with an estimated 1.6 million new cases and over 500,000 deaths per year. A key document summarizes evidence from several studies showing that the addition of ovarian suppression/ablation through drugs like Zoladex to standard therapies improves outcomes for premenopausal breast cancer patients. Specifically, it improves disease-free survival when added to chemotherapy for young premenopausal patients. It also improves disease-free and overall survival when given for 2 years after chemotherapy compared to chemotherapy alone in high-risk premenopausal breast cancer patients.