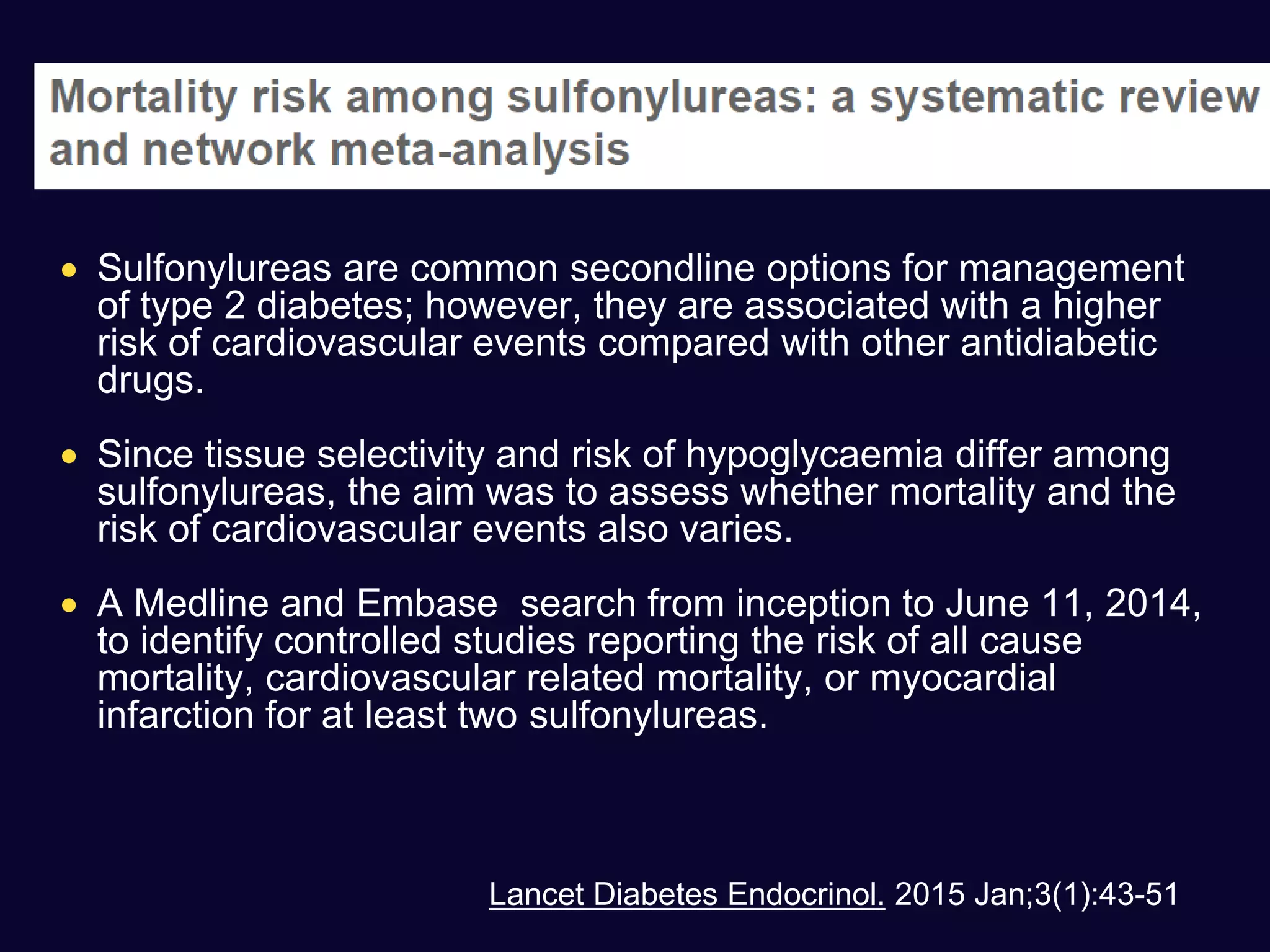
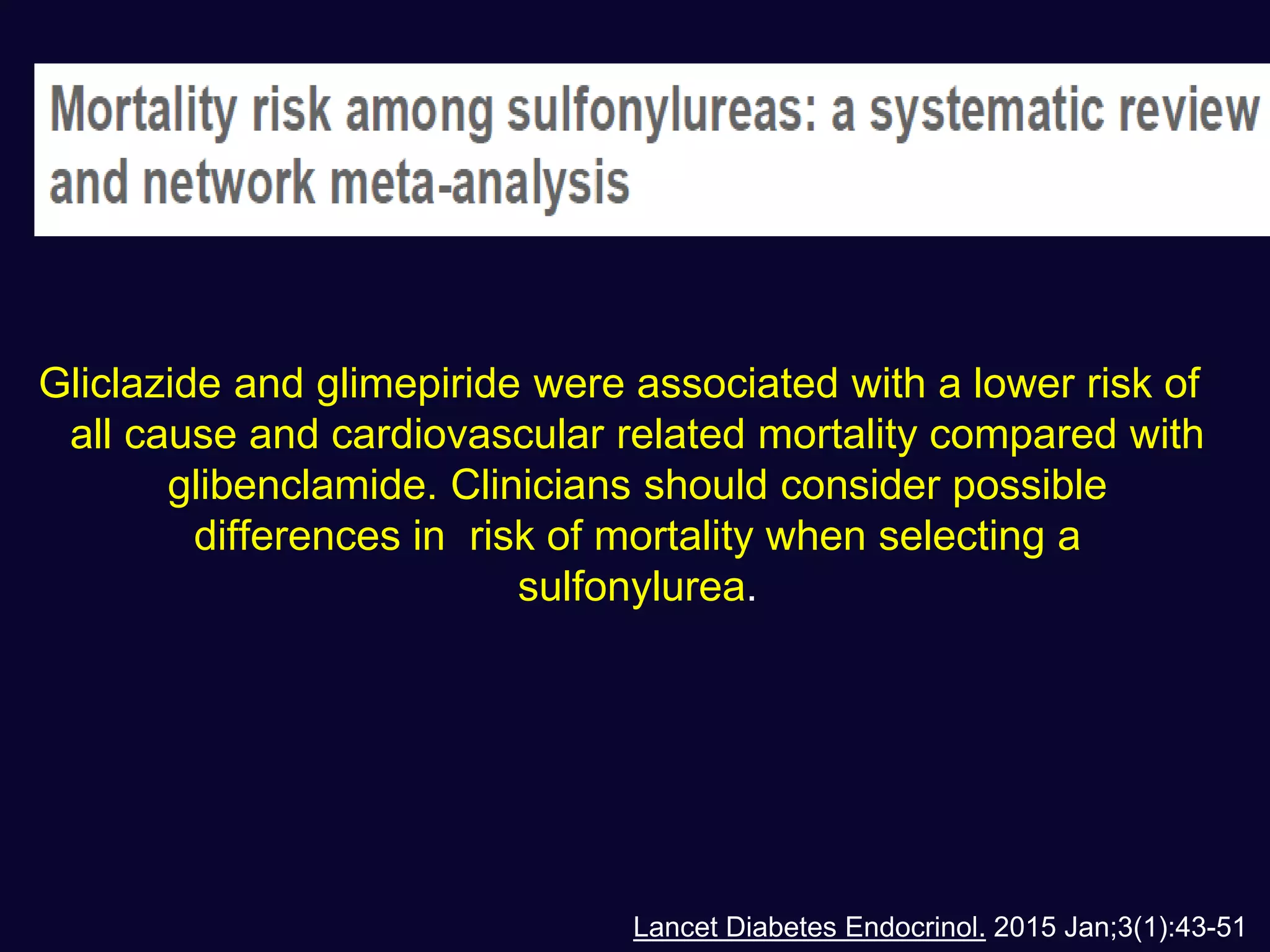
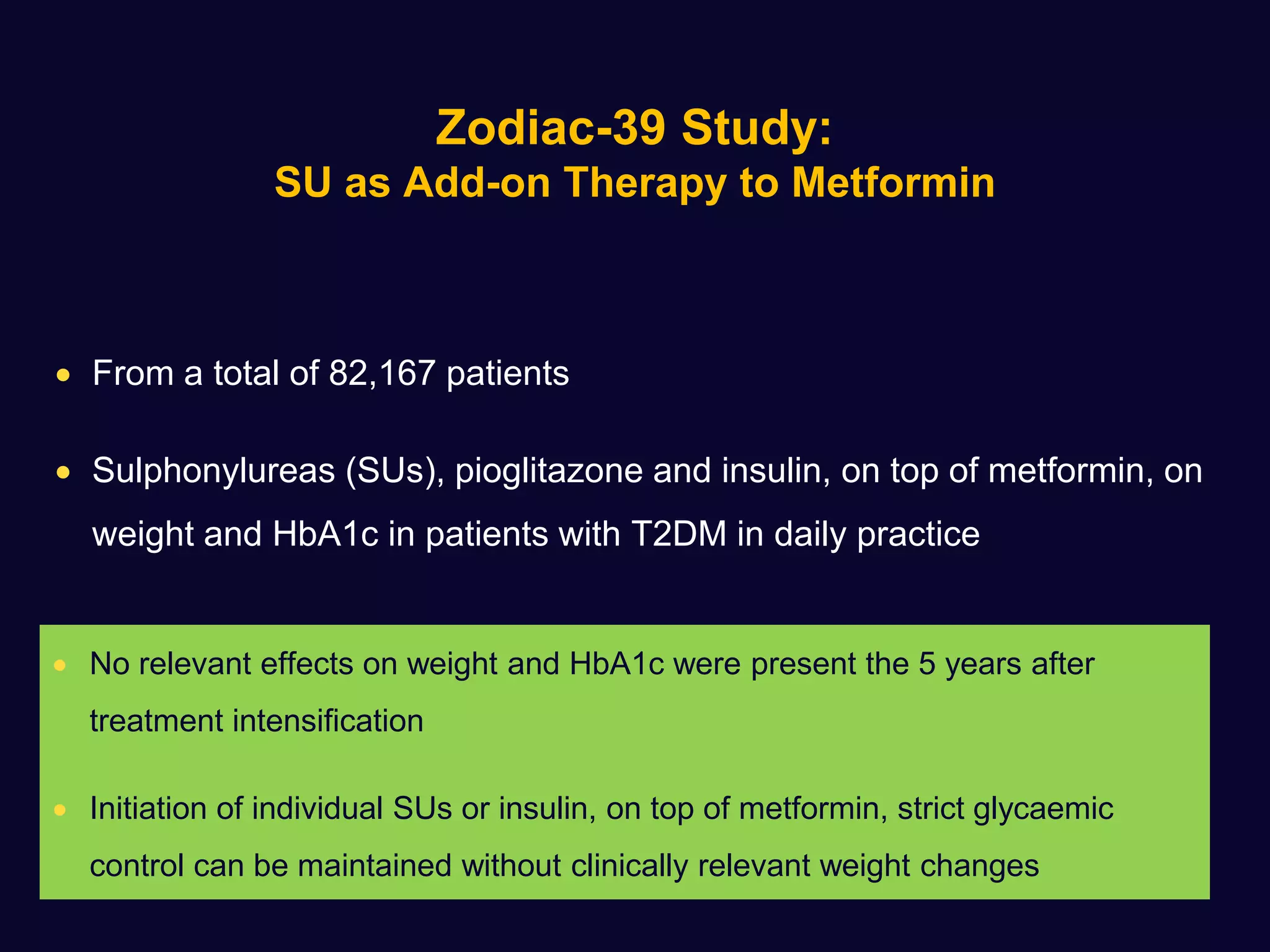
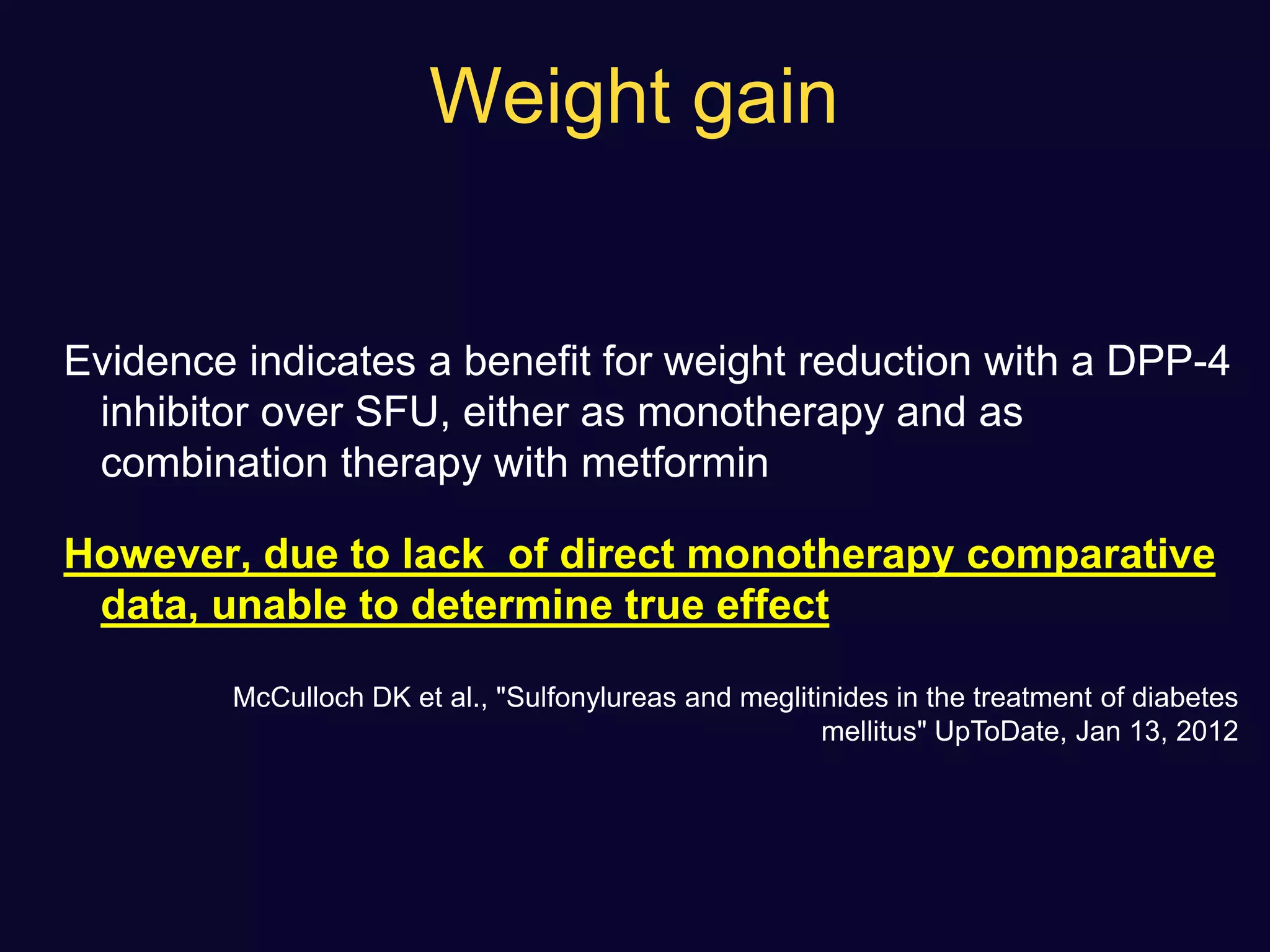
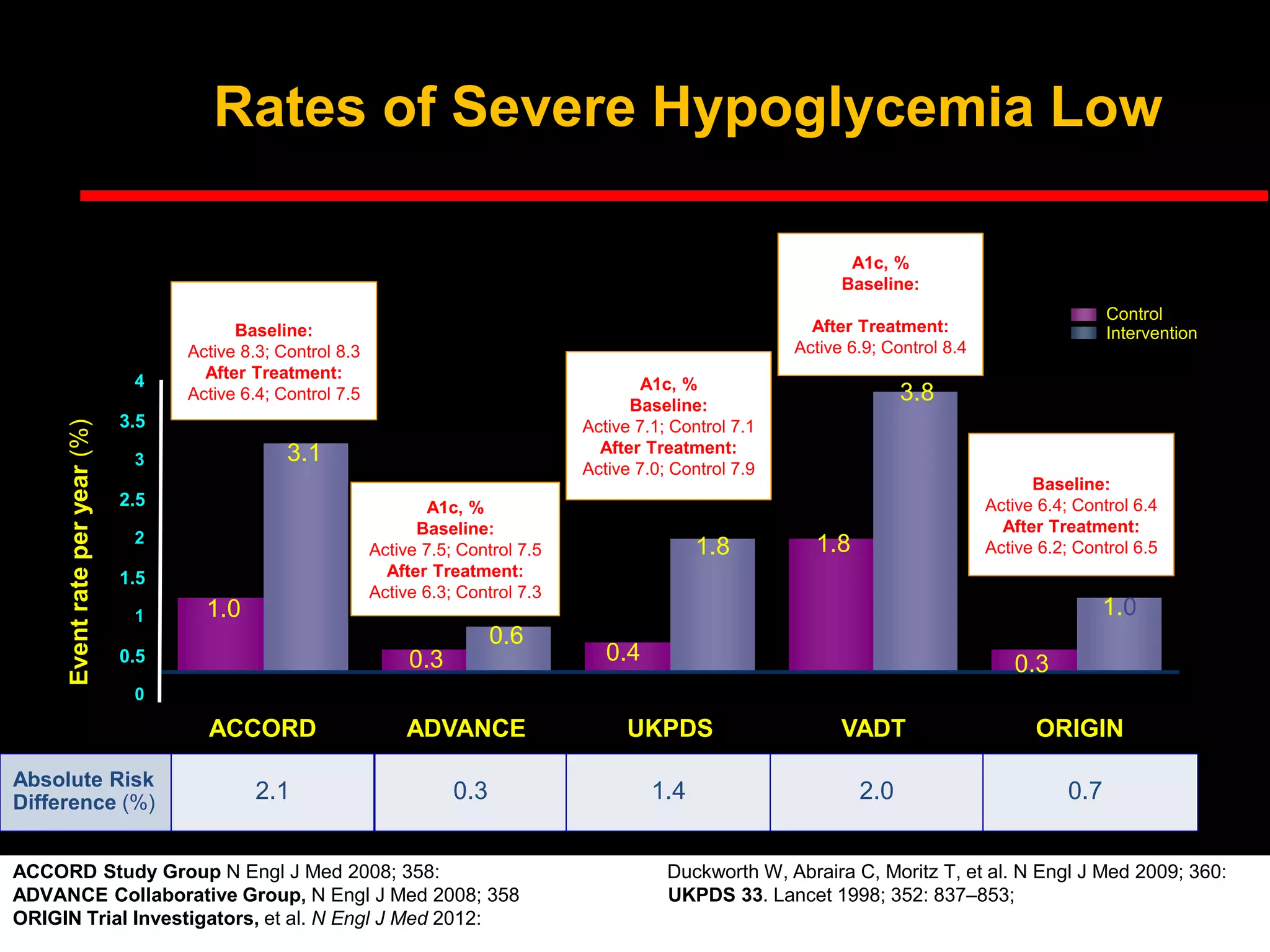
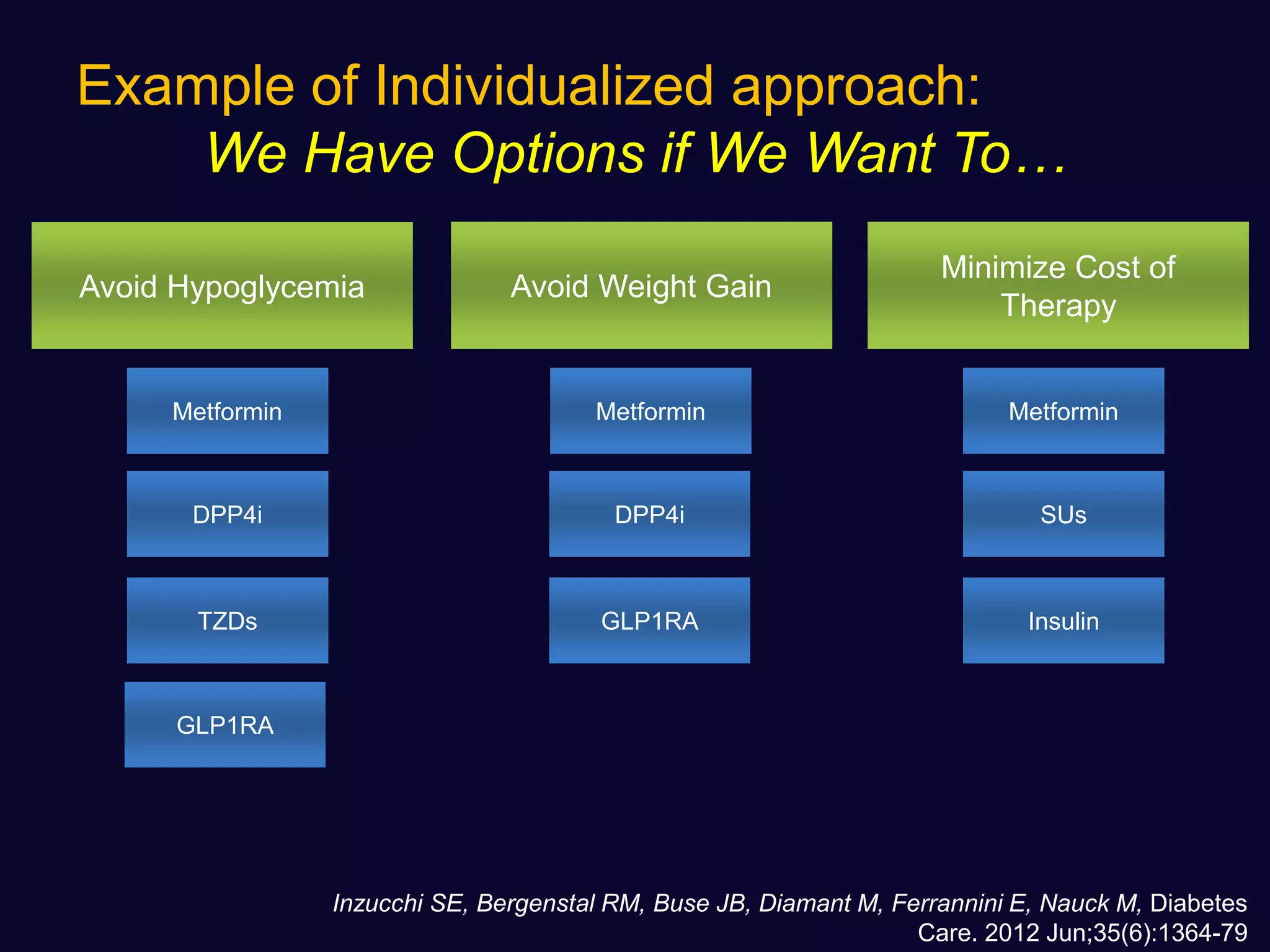
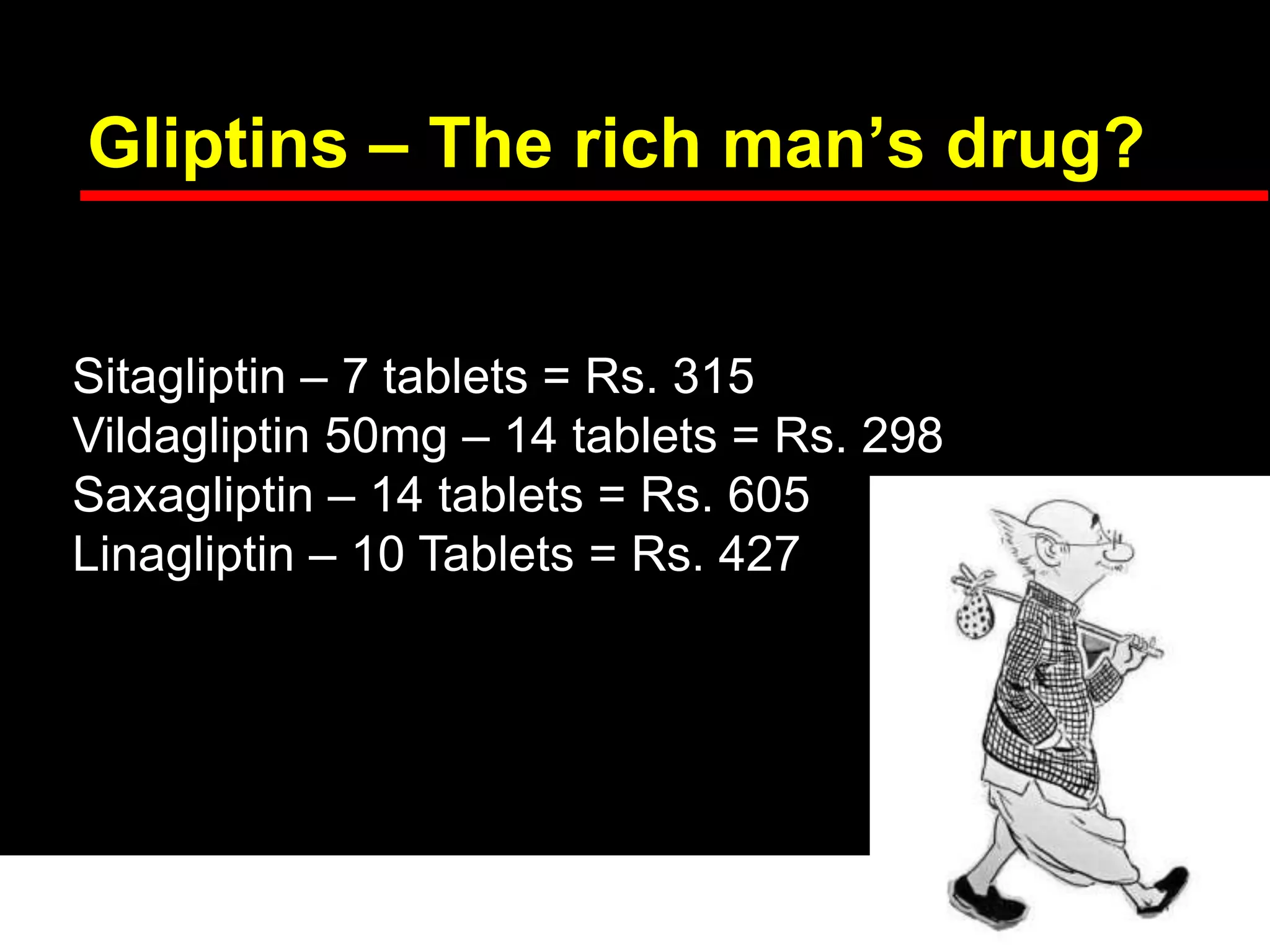
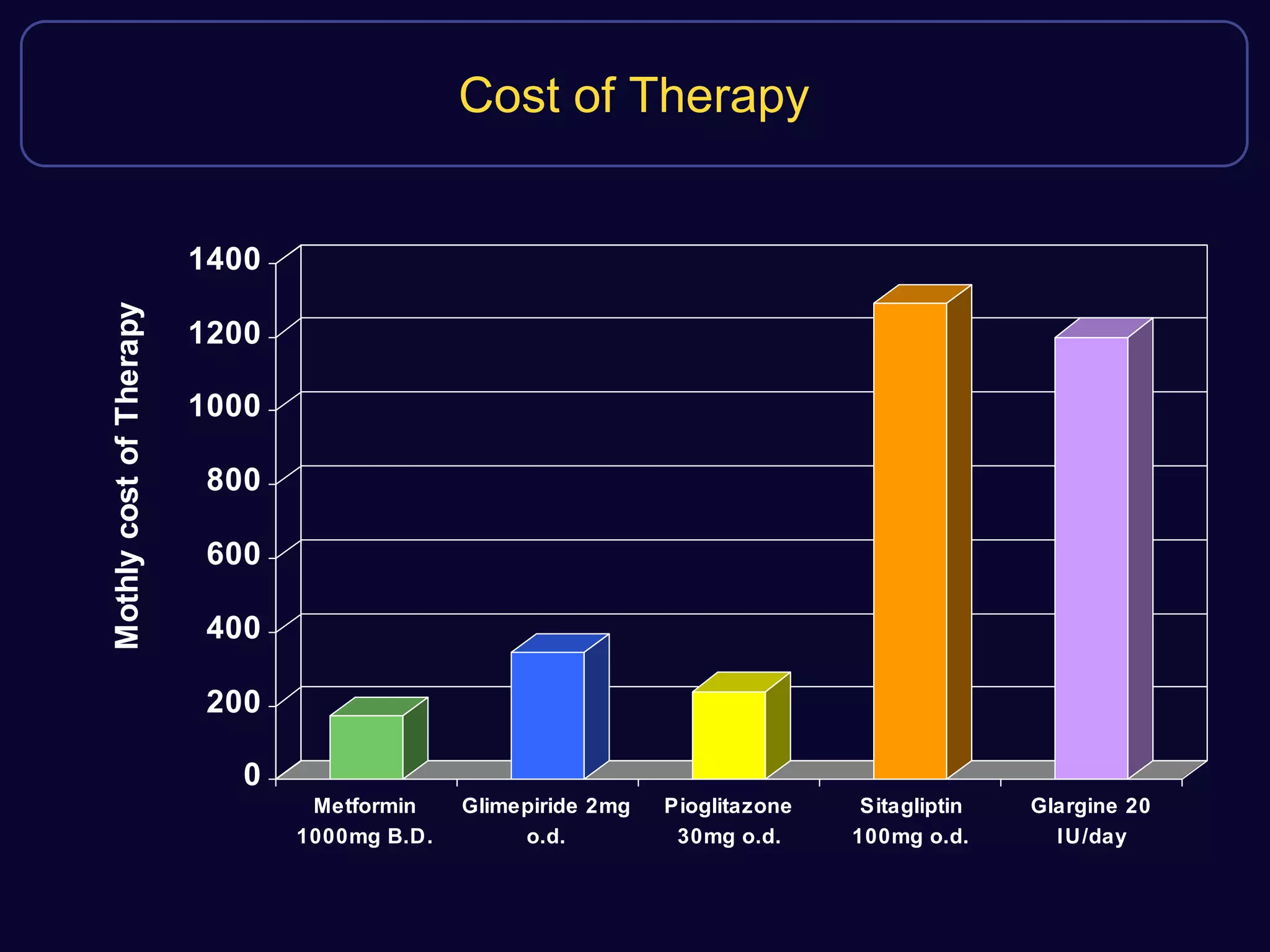
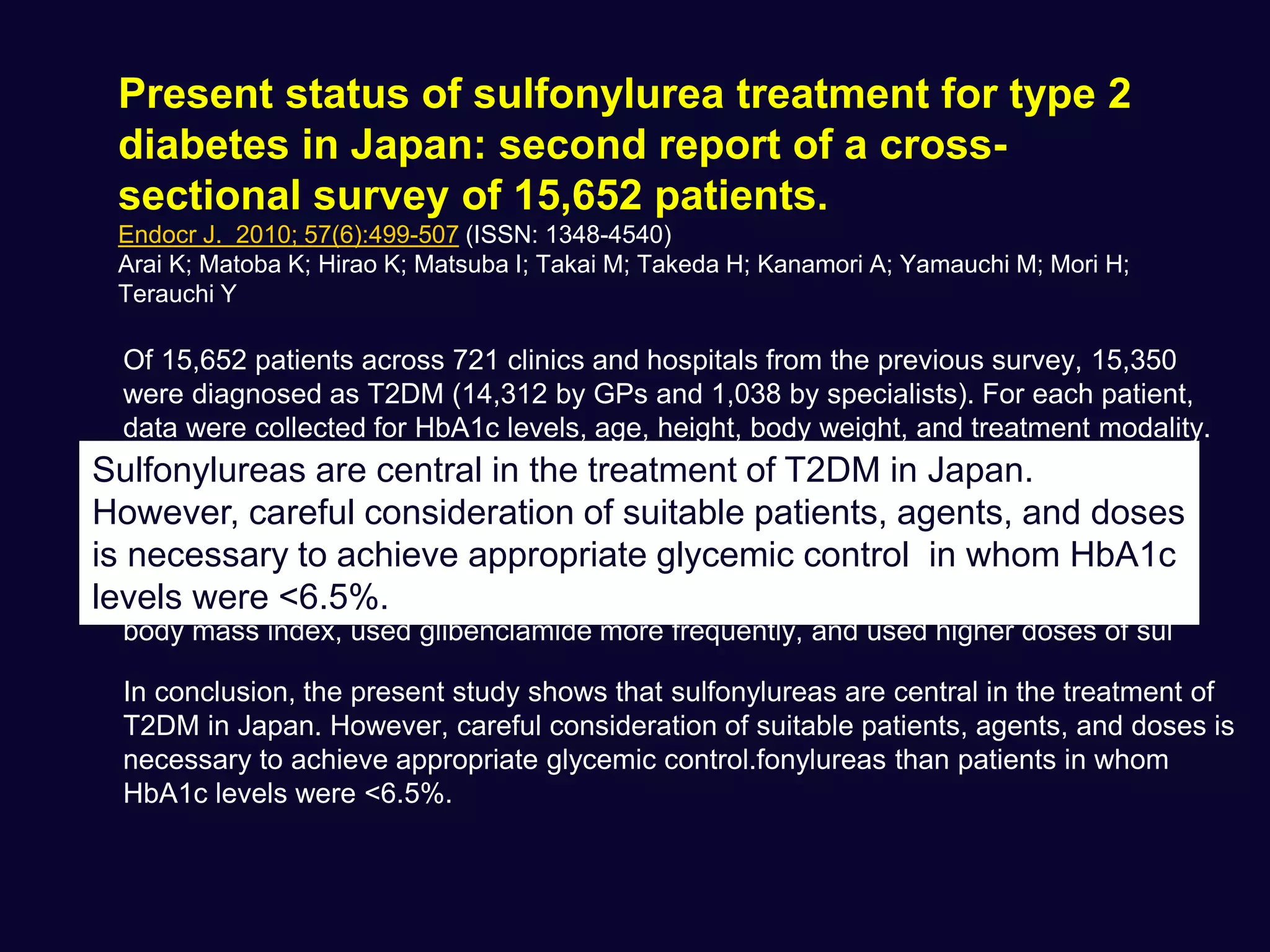
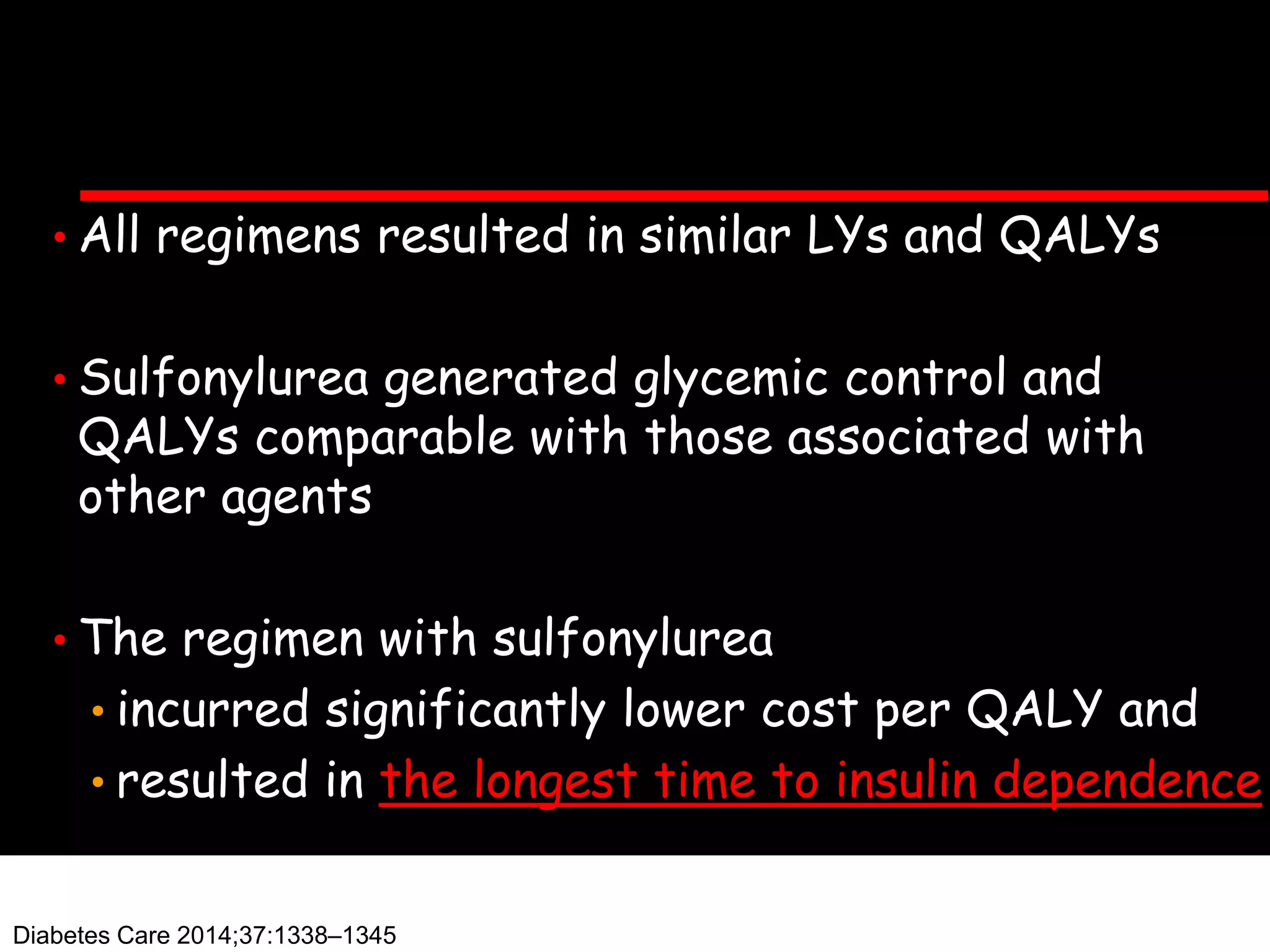
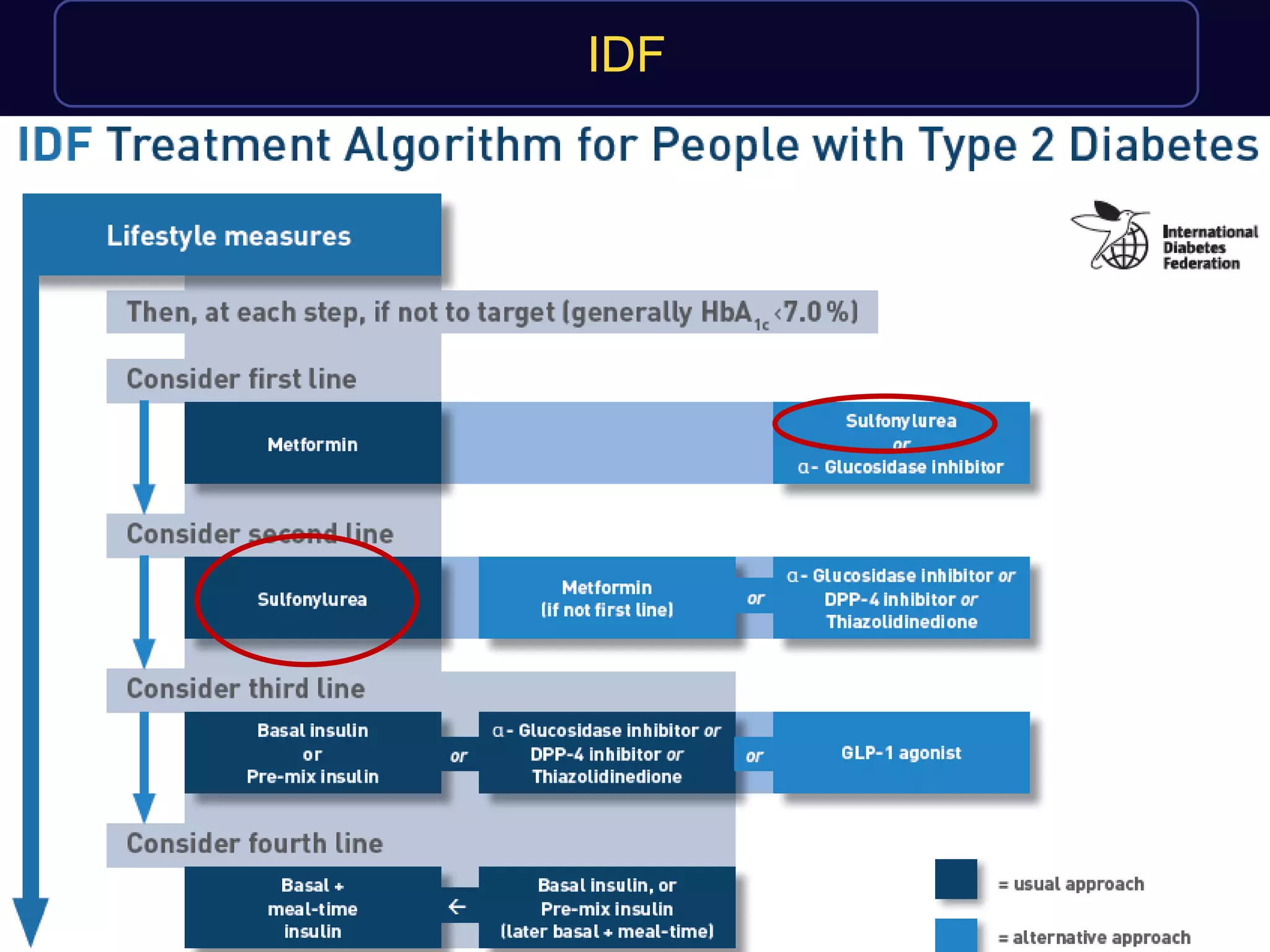
This document discusses treatment options after metformin for type 2 diabetes, comparing sulfonylureas and gliptins. It provides the following key points:
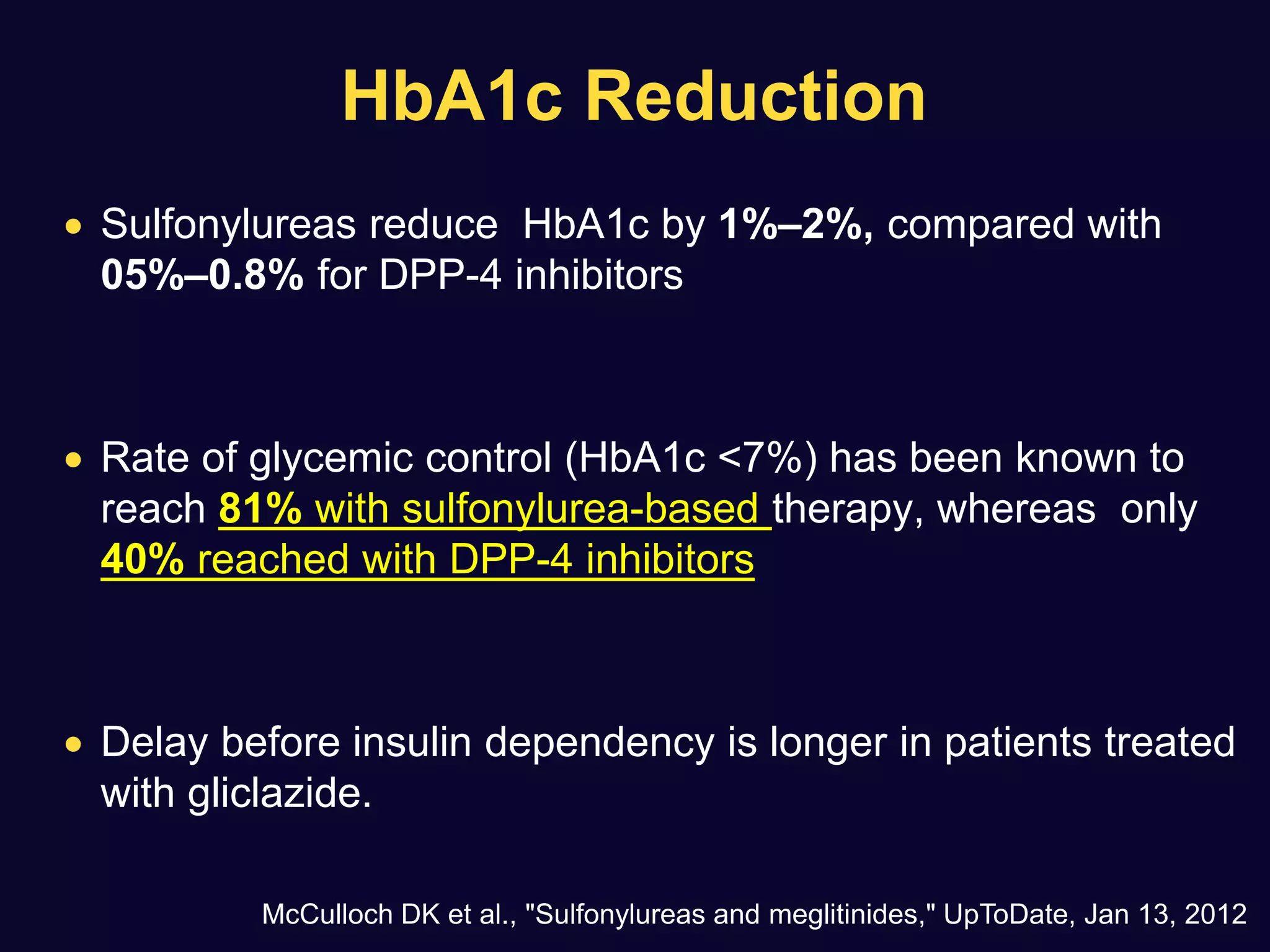
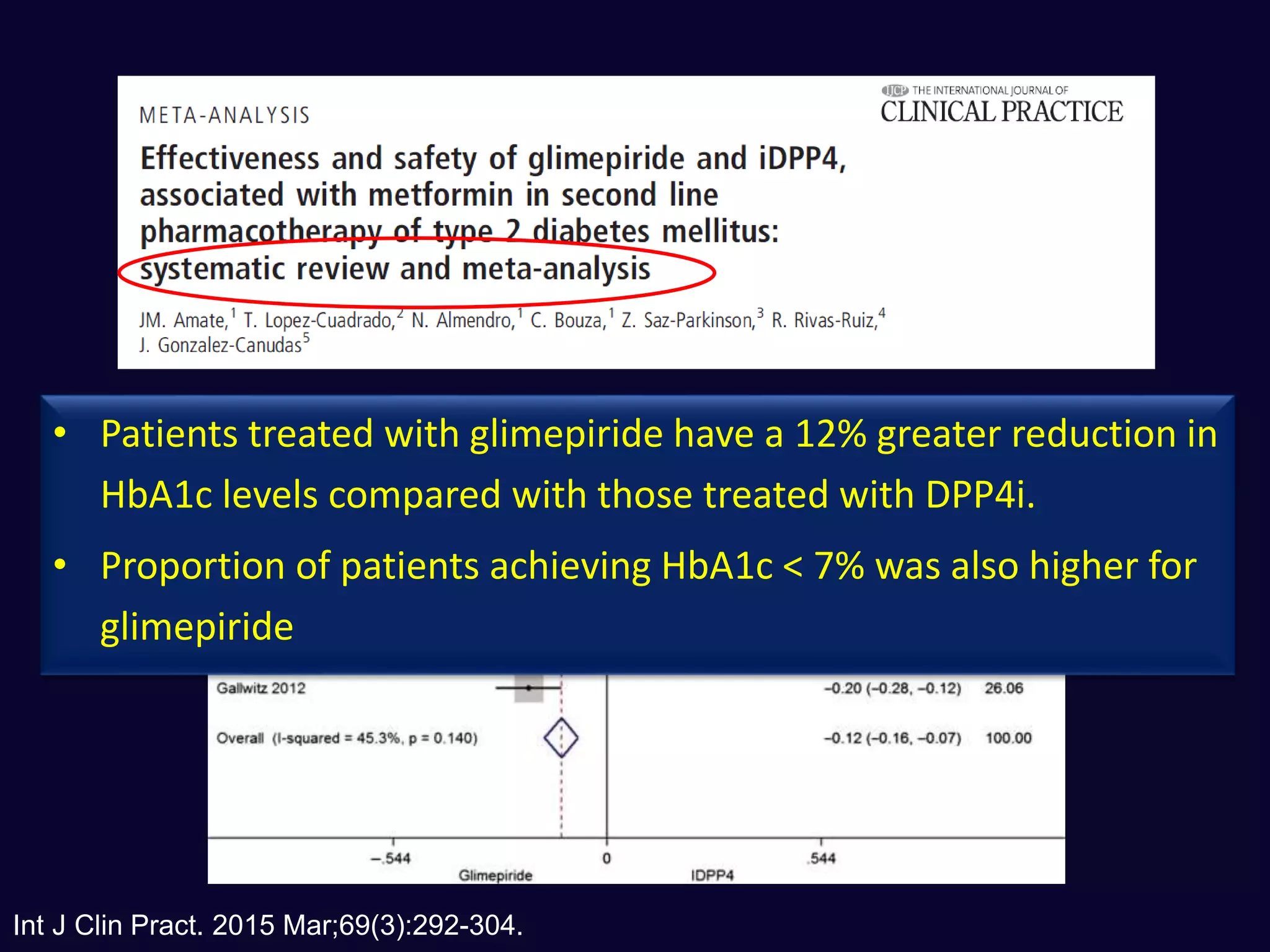
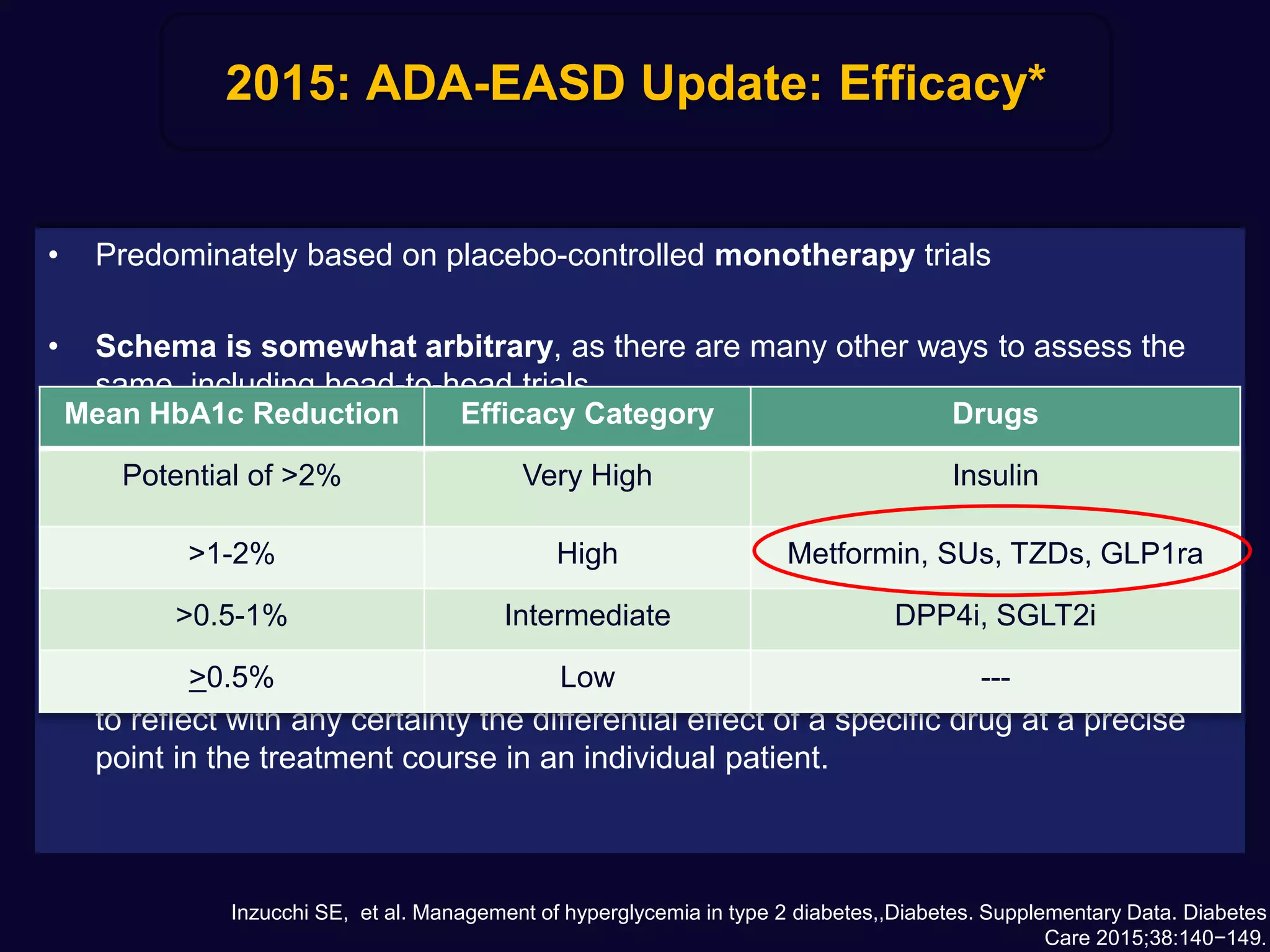
1. Sulfonylureas are more effective at reducing HbA1c levels and achieving glycemic control targets compared to gliptins. They lower HbA1c by 1-2% on average versus 0.5-1% for gliptins.
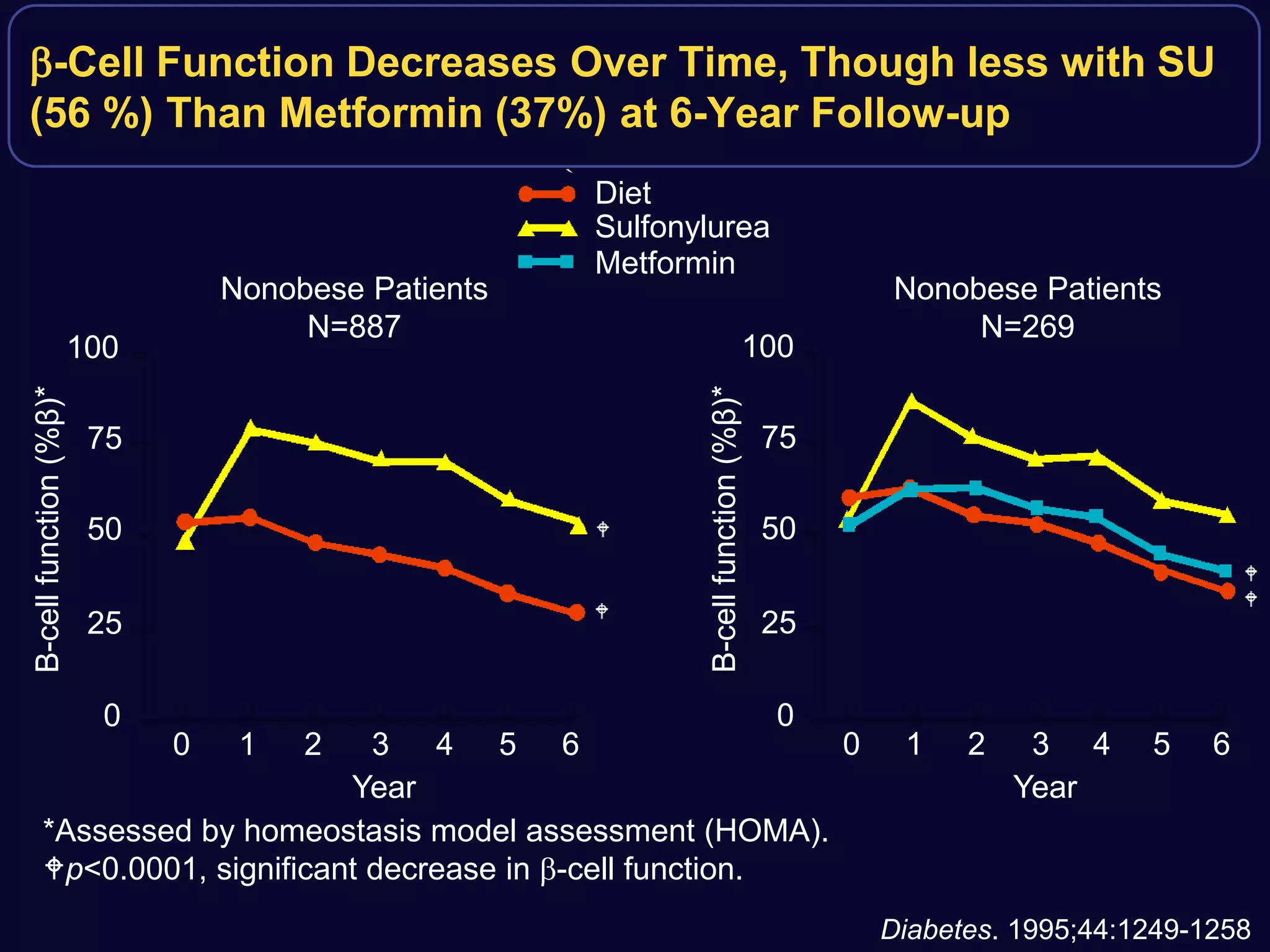
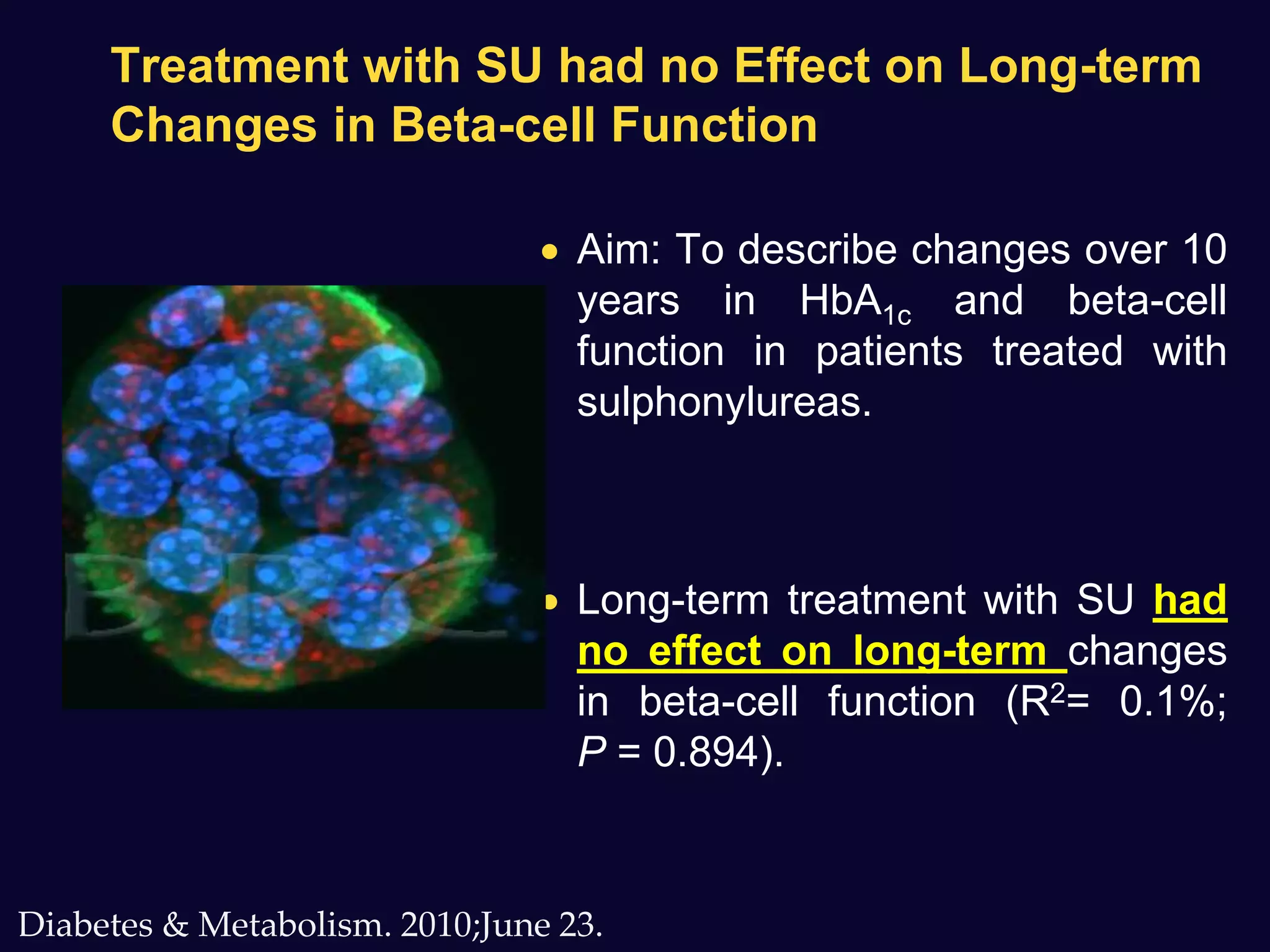
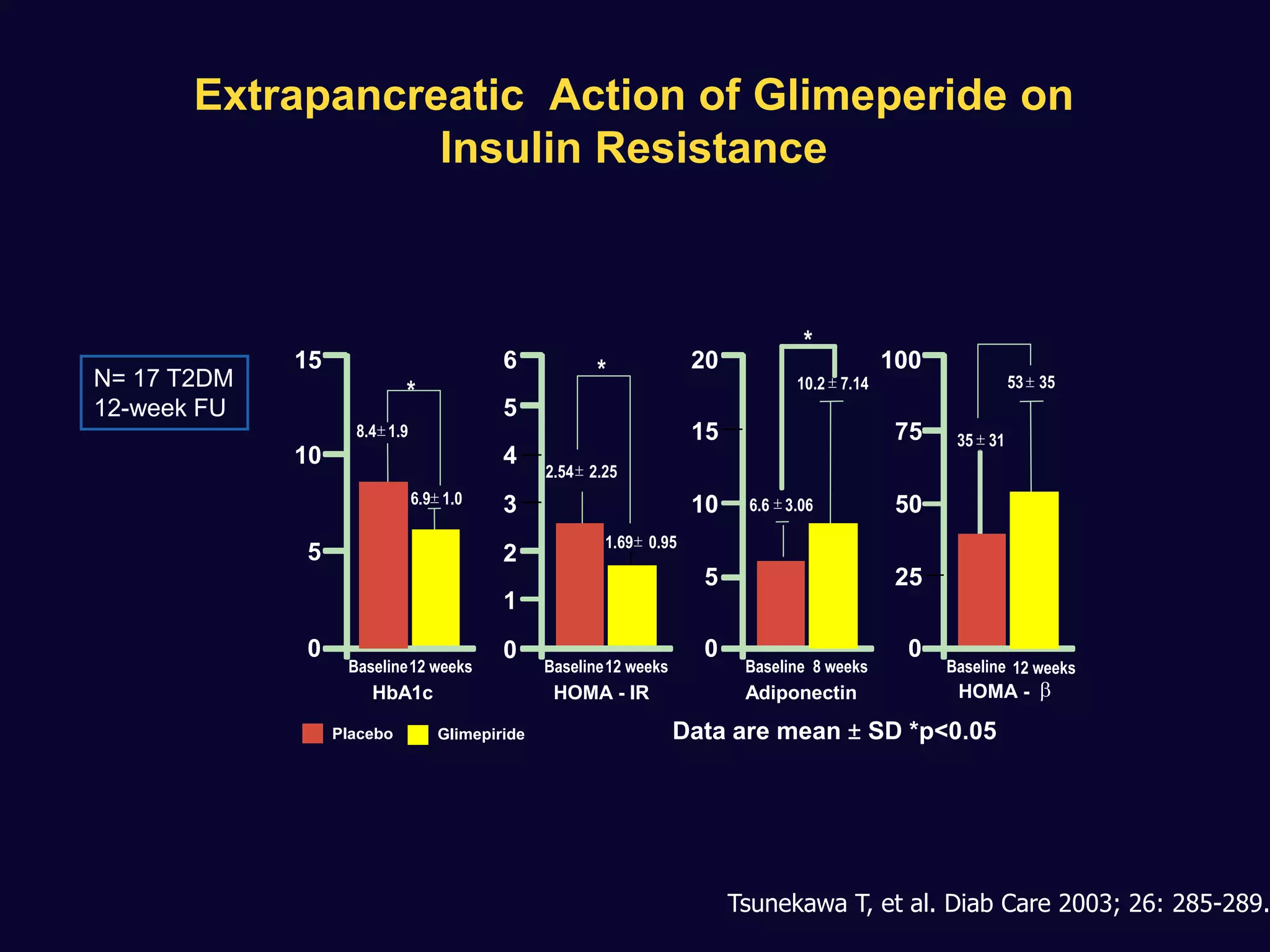
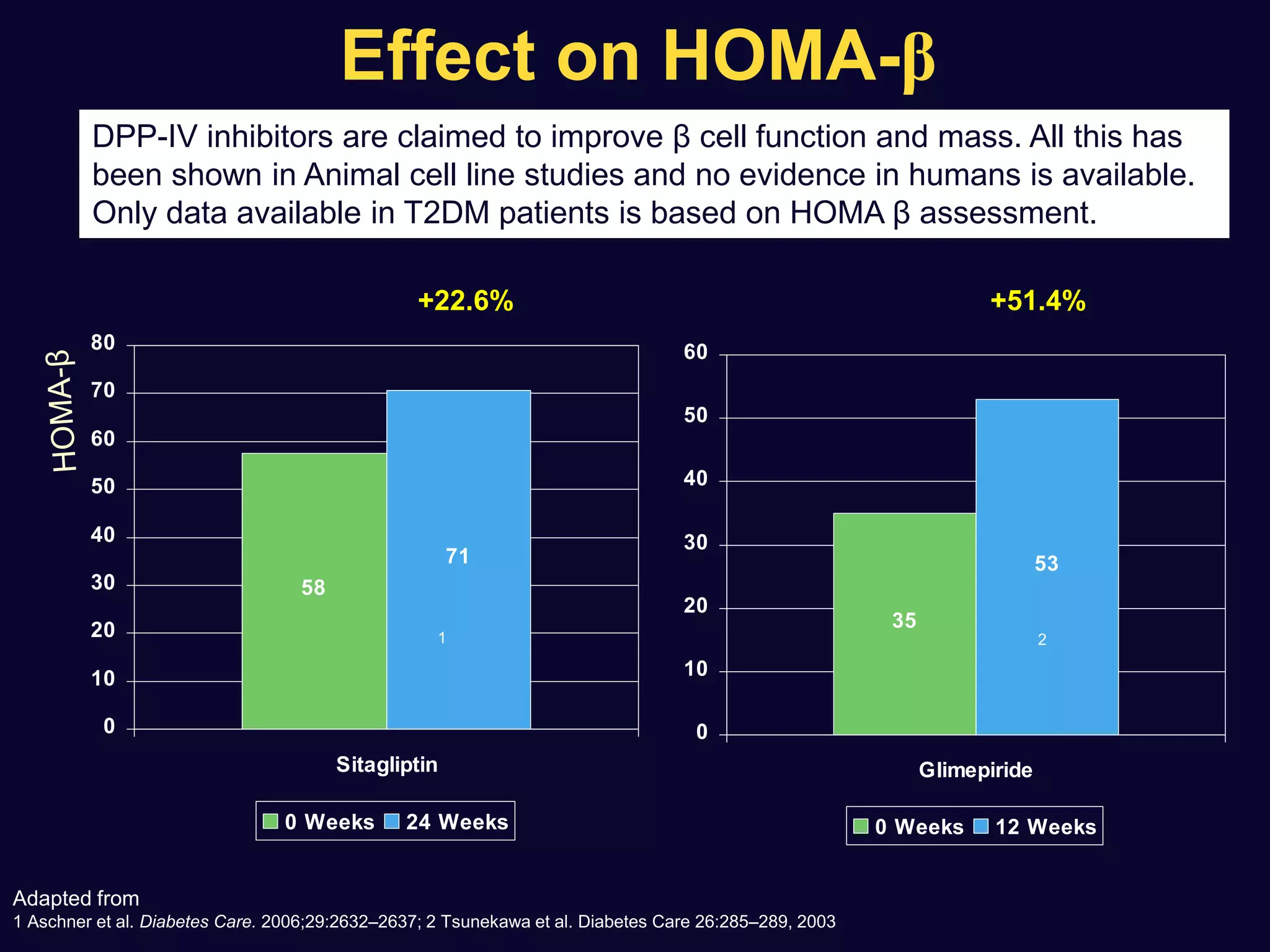
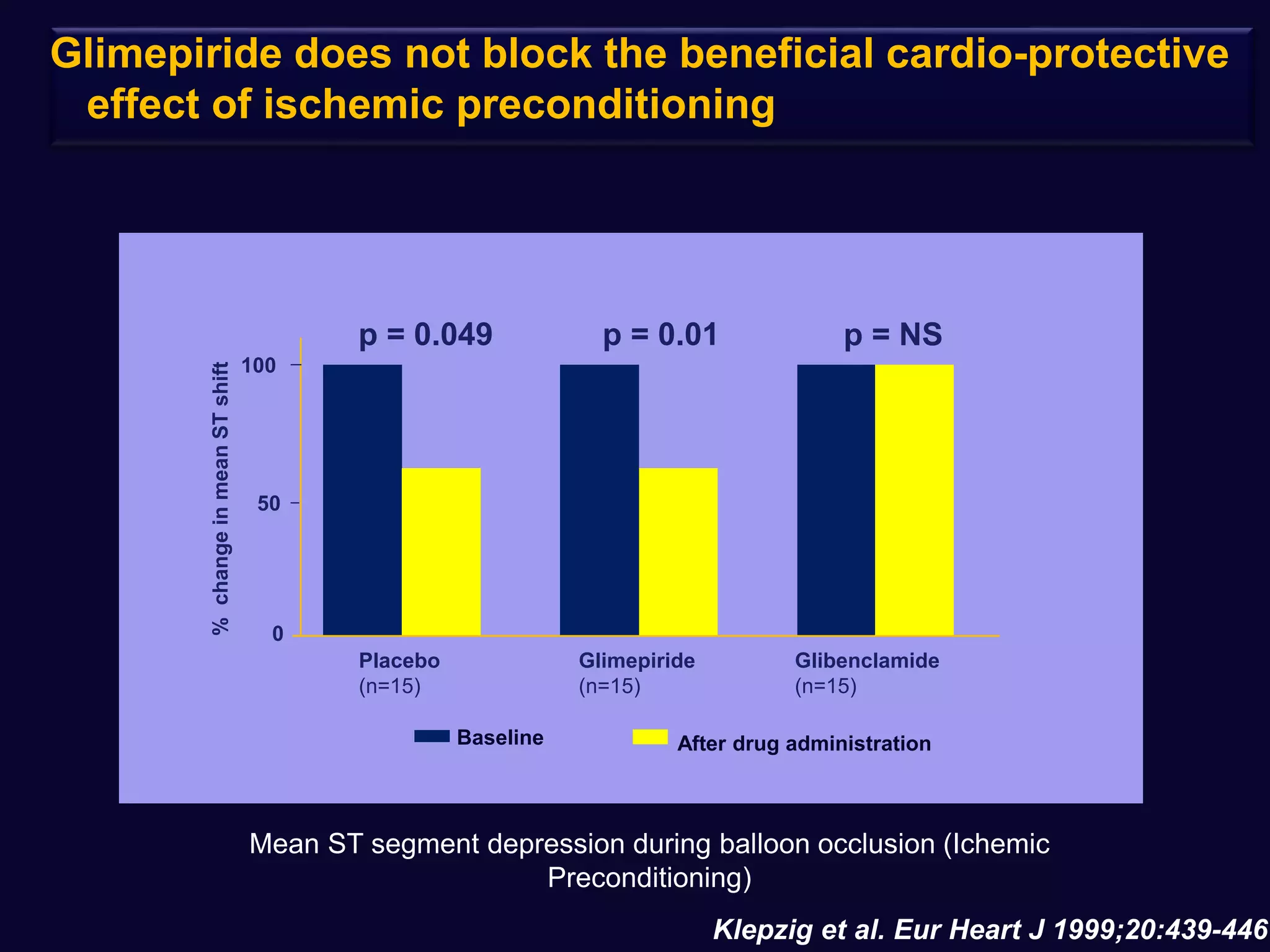
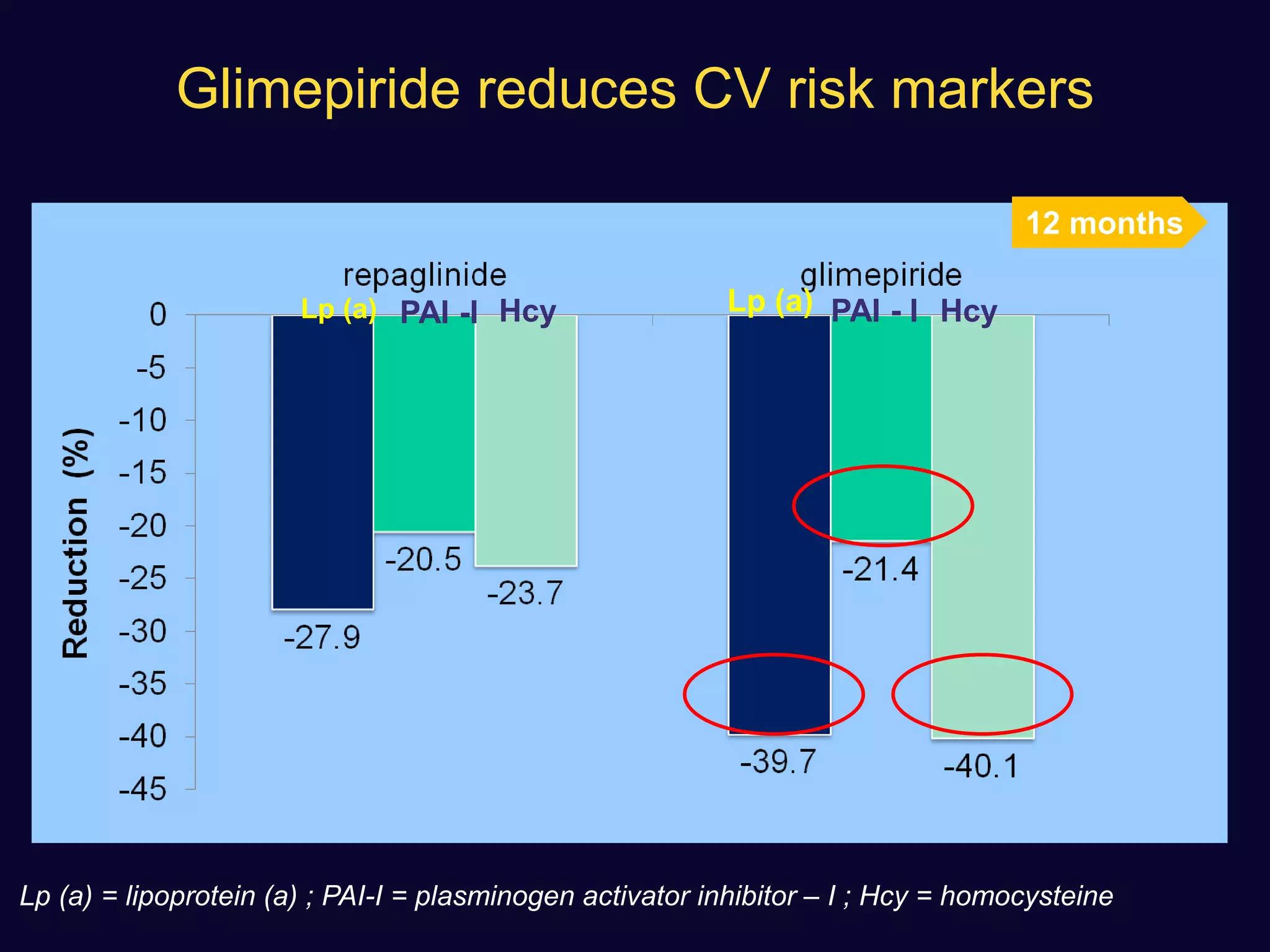
2. Studies show sulfonylureas may better preserve beta-cell function in the long-term compared to metformin or gliptins. They have been shown to enhance insulin secretion from beta and alpha cells.
3. While older studies linked sulfonylureas






![Rosenstock et al. 2013
Arjona-Ferreira et al. 2013
a
Arjona-Ferreira et al. 2013
b
Nauck et al. 2007
Arechavaleta et al. 2011
Foley &
Sreenan
2009
Ferrannini et al. 2009
Filozof &
Gautier 2010
Gause-Nilsson
et al. 2010
Gallwitz et al. 2012
-1.0
-0.9
-0.8
-0.7
-0.6
-0.5
-0.4
-0.3
-0.2
-0.1
Baseline HbA1c:
7.5 7.8 7.9 7.3 7.5
Glimepiride
Linagliptin
Sitagliptin
Glipizide
8.6 8.5 7.7 7.7
Saxagliptin
Alogliptin
Vildagliptin
Gliclazide
7.7
Renal functional
impairment
Metformin
background
HbA
1c
[%]
DPP-4 Inhibitors vs. Sulfonylureas as
Monotherapy or Add-On to Metformin](https://image.slidesharecdn.com/whatnextaftermetformindpp4vssu-210405012511/75/What-next-after-metformin-dpp4-vs-su-10-2048.jpg)
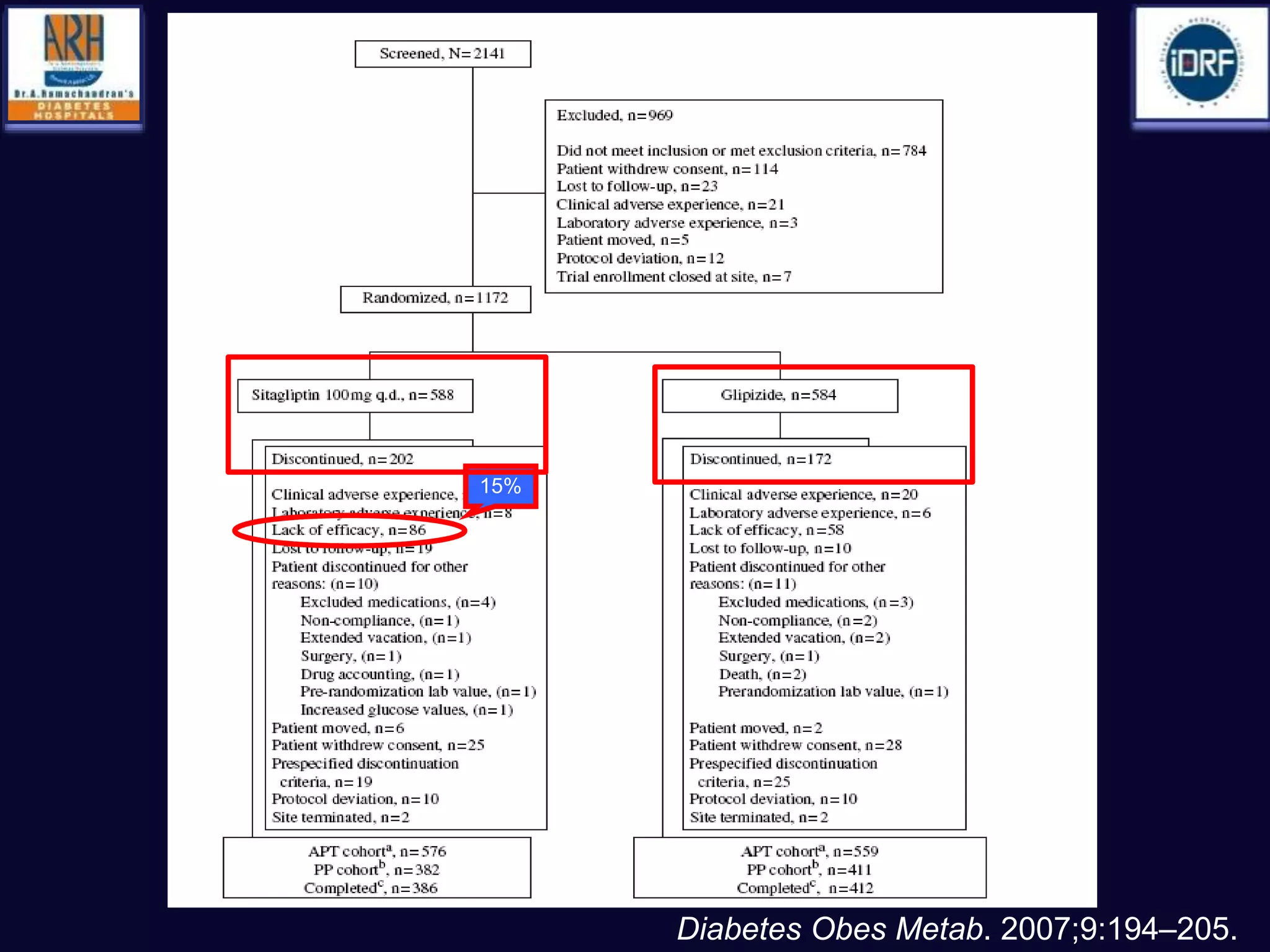









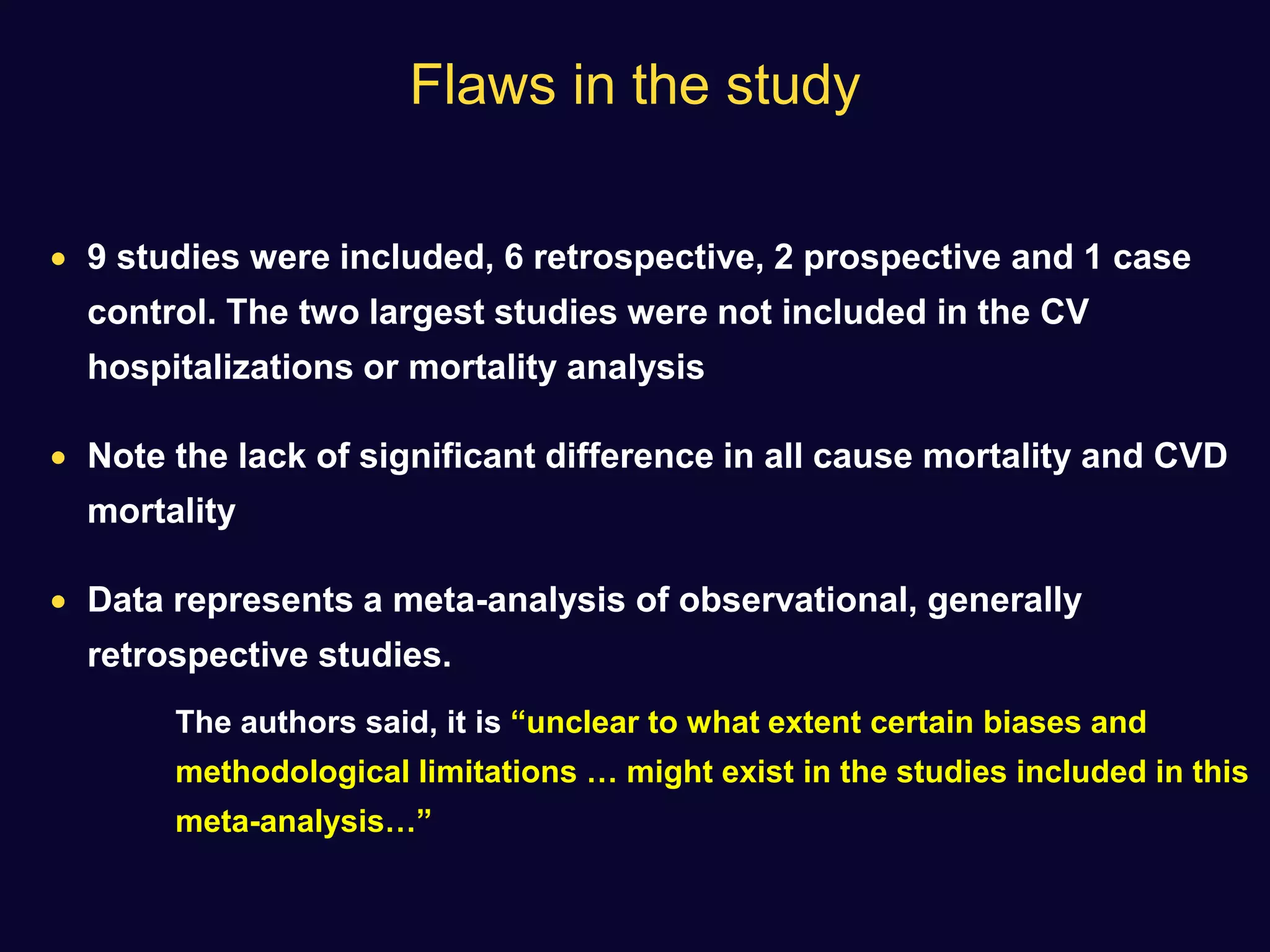

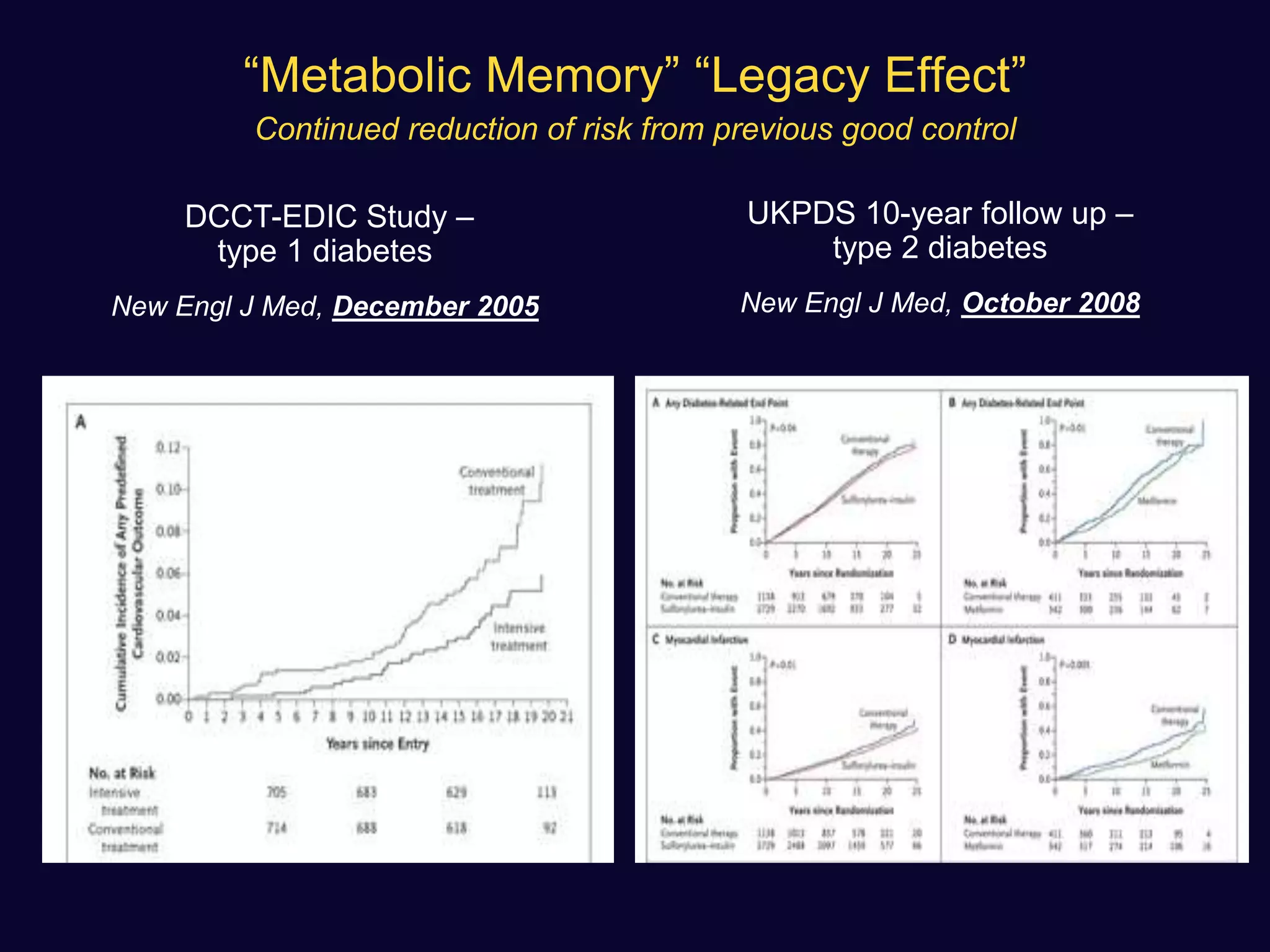
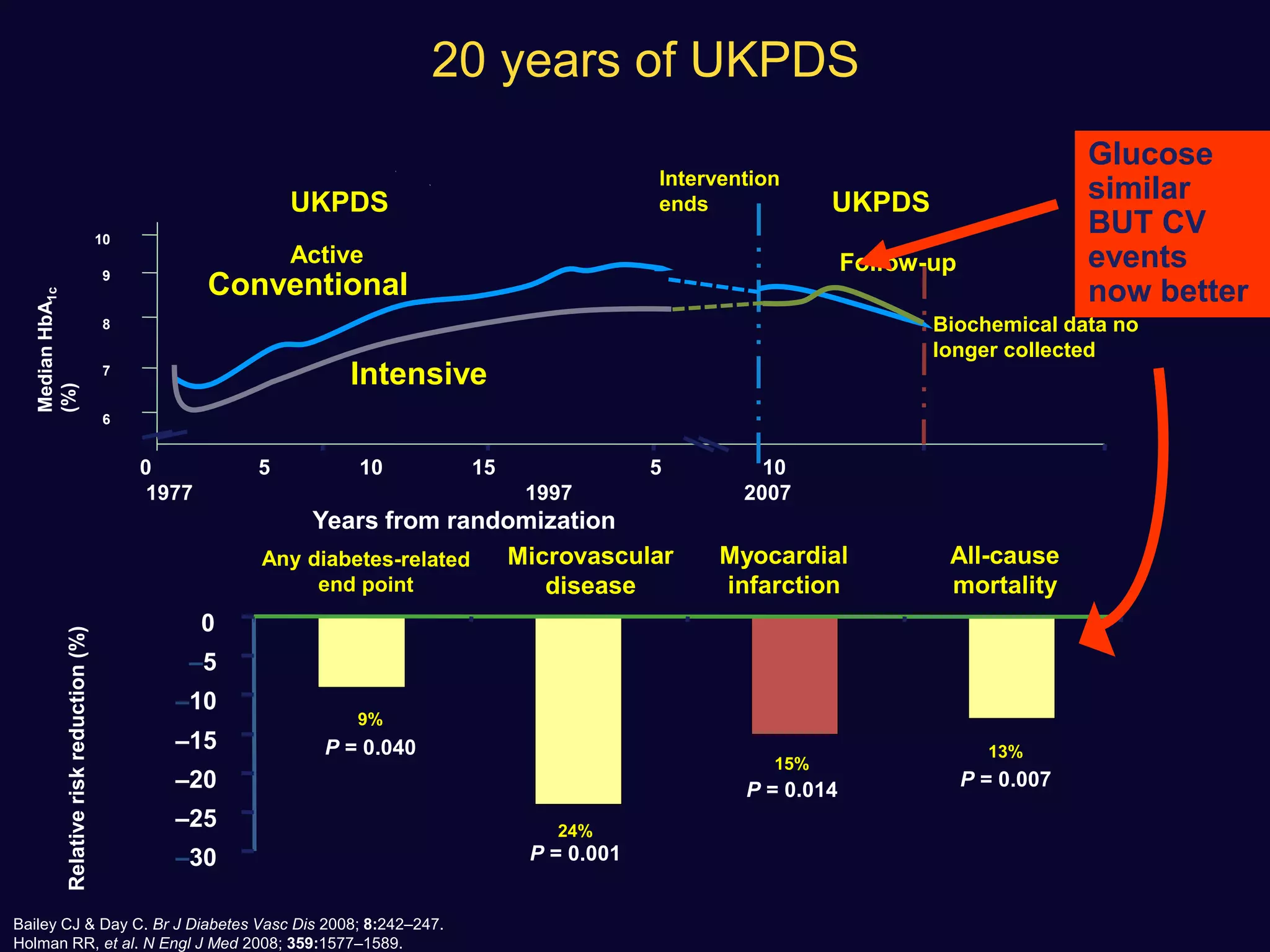
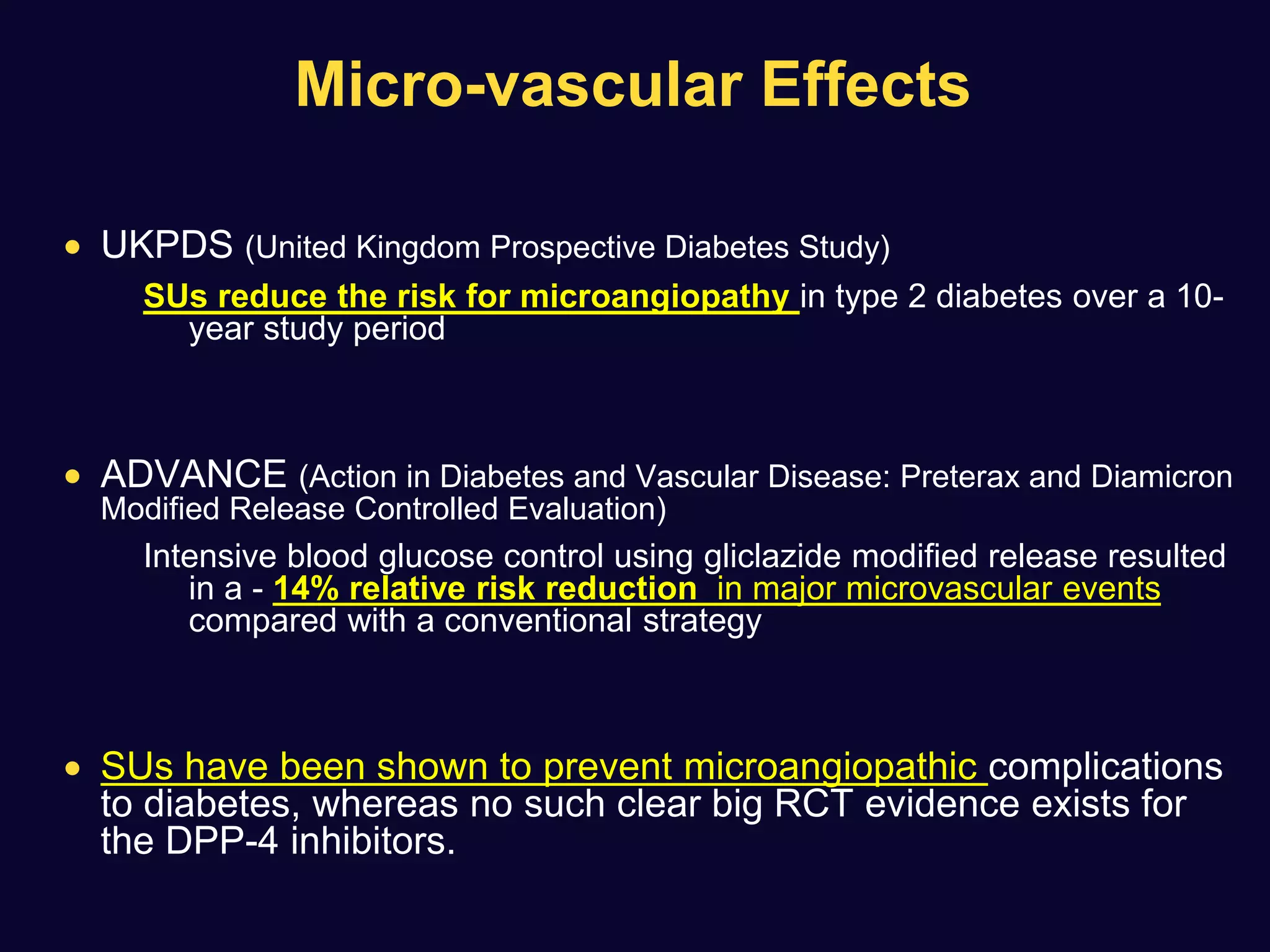

![ Use of SU not associated with any significant difference in the incidence
of MI with respect to comparators (OR: 0.88 [0.75–1.04], p=0.13)
A non-significant trend towards a reduction was observed in
comparison with placebo or no therapy.
None of the sulfonylureas appeared to affect the incidence of MI
Diabetes, Obesity and Metabolism 15: 938–953, 2013.](https://image.slidesharecdn.com/whatnextaftermetformindpp4vssu-210405012511/75/What-next-after-metformin-dpp4-vs-su-36-2048.jpg)