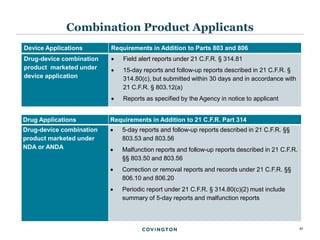
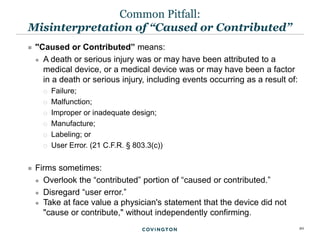
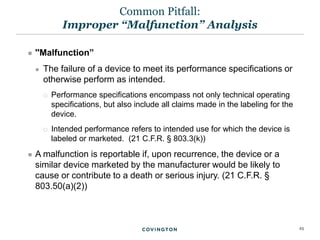
The document discusses best practices for medical device reporting (MDR) according to FDA regulations. It covers an overview of the MDR reporting requirements, common pitfalls manufacturers face in complying with MDR, and FDA enforcement trends. The presentation agenda includes an overview of the MDR regulations and guidance, common MDR reporting issues such as what constitutes a complaint, and FDA's rules for adverse event reporting for combination products.


















![19
Warning Letter Example
“[O]n July 27, 2015, your firm became aware of an event
…Your firm did submit an MDR related to this event...,
received by FDA on September 23, 2015. However, this MDR
was not submitted within the required 30 calendar day reporting
timeframe. “](https://image.slidesharecdn.com/webinarpresentationslides-230213113305-184c90d8/85/Webinar-Presentation-Slides-pdf-19-320.jpg)










![32
Warning Letter Example
“[Y]our firm submitted 33 MDRs to the FDA that did not
identify the "Date of event" in Block B3 of the FDA Form
3500A. In addition, your firm did not include in Block H11-
"Corrected data", of the associated 3500A forms, an explanation
of why the required information was not provided and the steps
taken to obtain such information.”](https://image.slidesharecdn.com/webinarpresentationslides-230213113305-184c90d8/85/Webinar-Presentation-Slides-pdf-32-320.jpg)