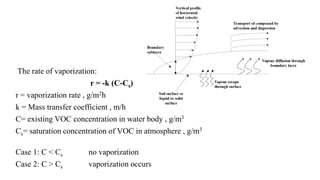
Volatilization is the process where solids and liquids vaporize into the atmosphere. There are three mechanisms: vapors escape the air/liquid interface, diffuse through the boundary layer via molecular and turbulent diffusion, and are transported away by advection and dispersion. The rate of vaporization depends on the mass transfer coefficient, existing contaminant concentration in water, and saturation concentration in air. If the concentration is below saturation, no vaporization occurs; above saturation, vaporization takes place. The loss of contaminants from a water body can be calculated using the rate, surface area, depth, and change in concentration over time.