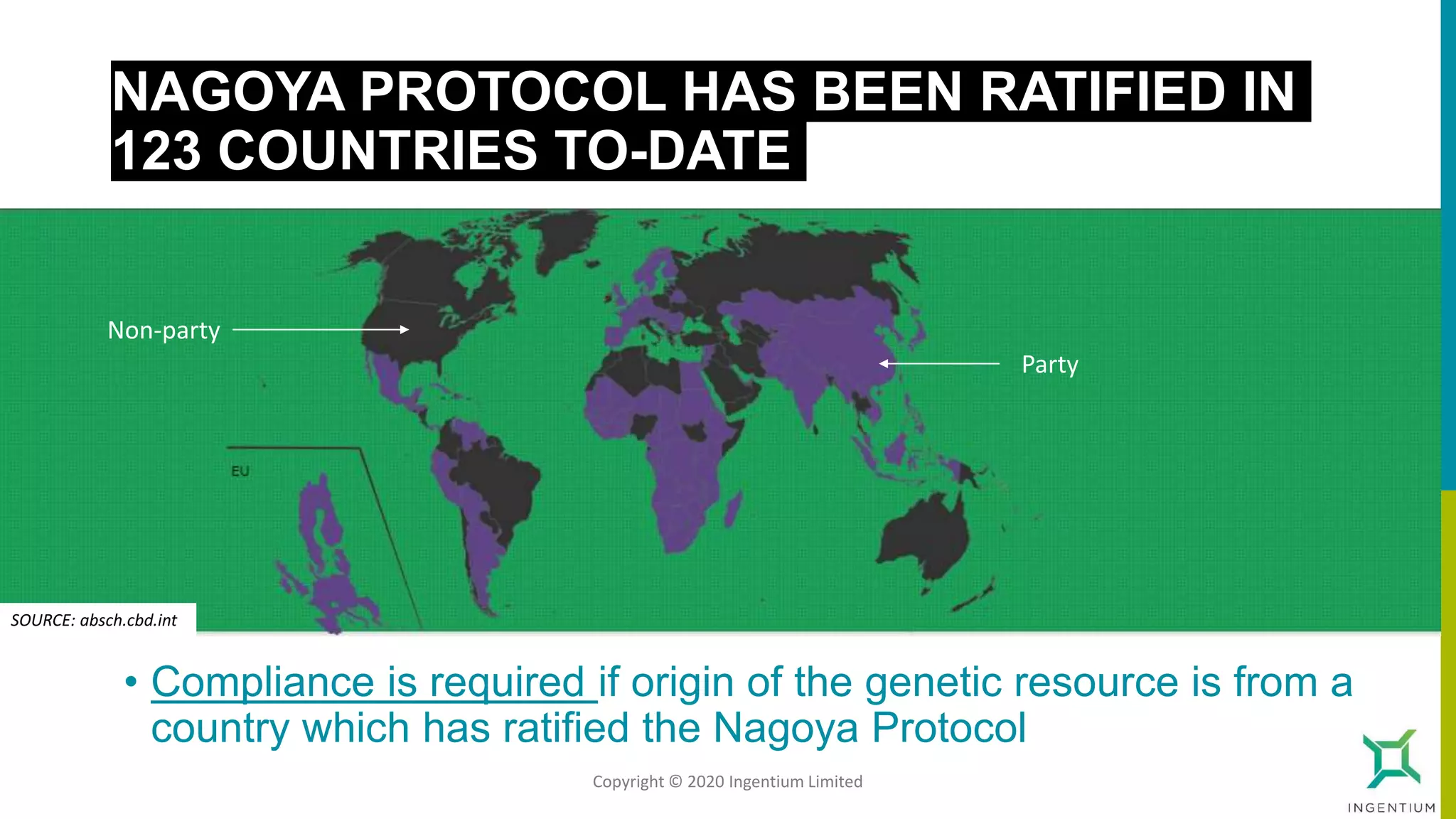
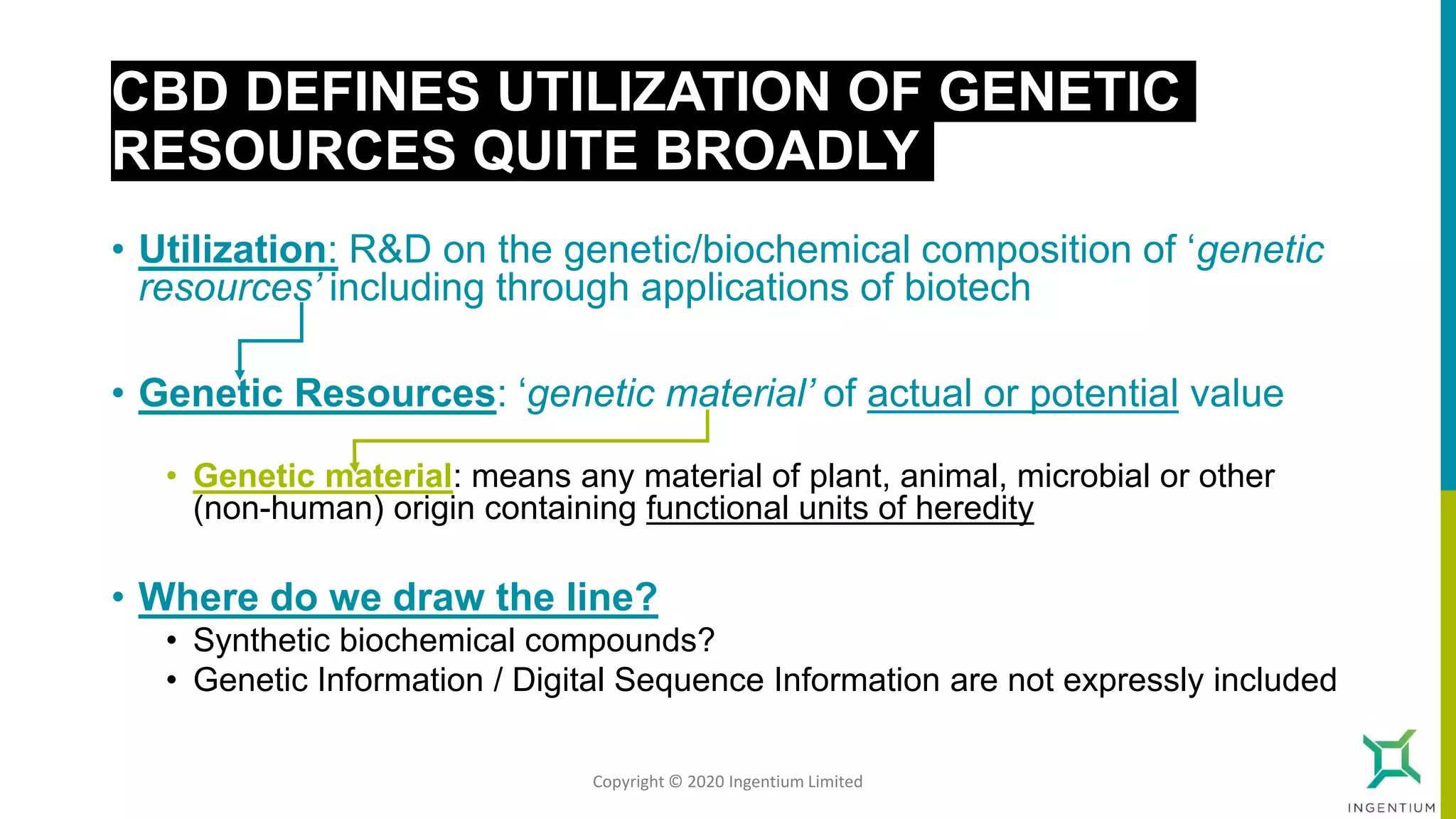
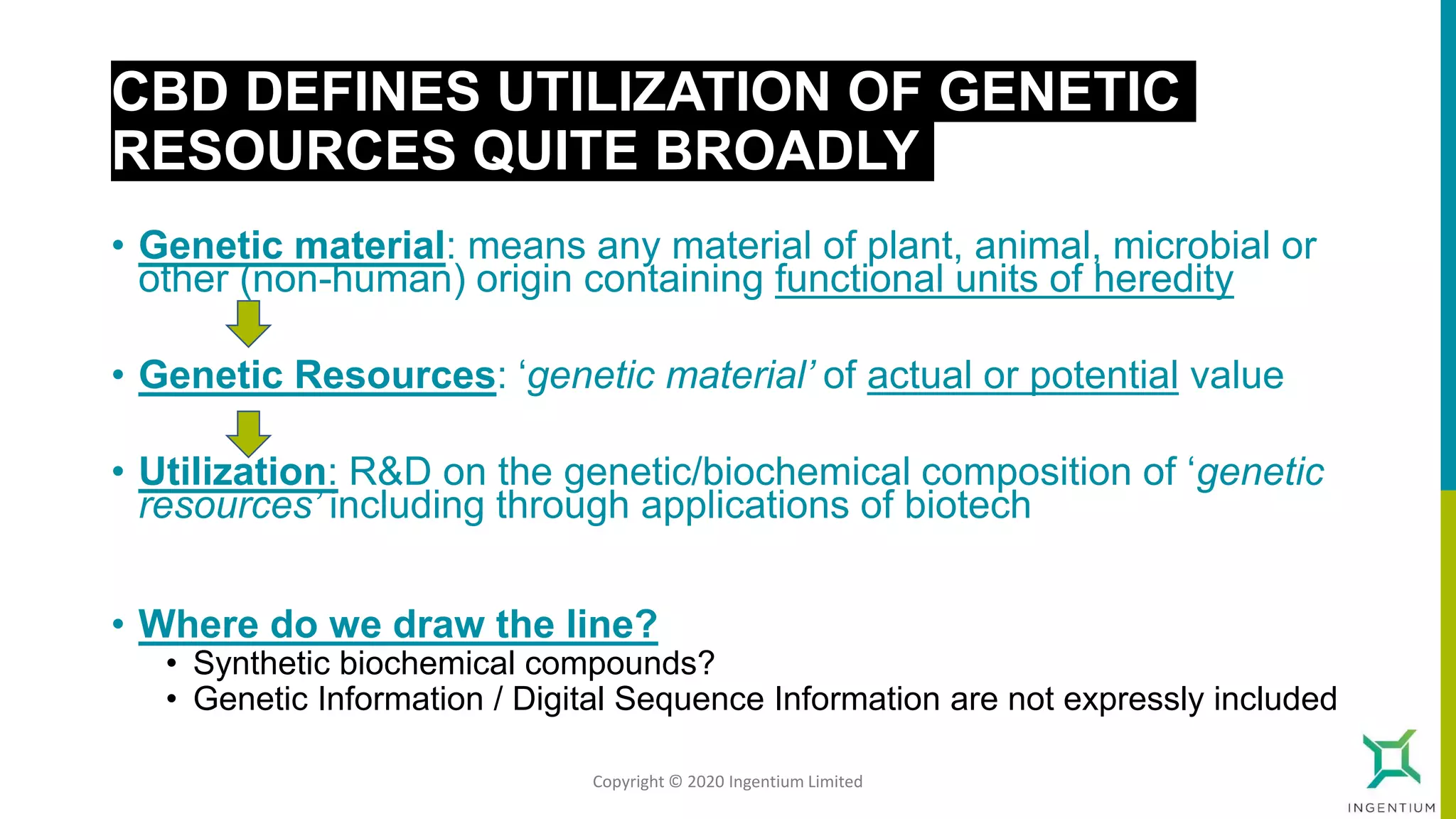
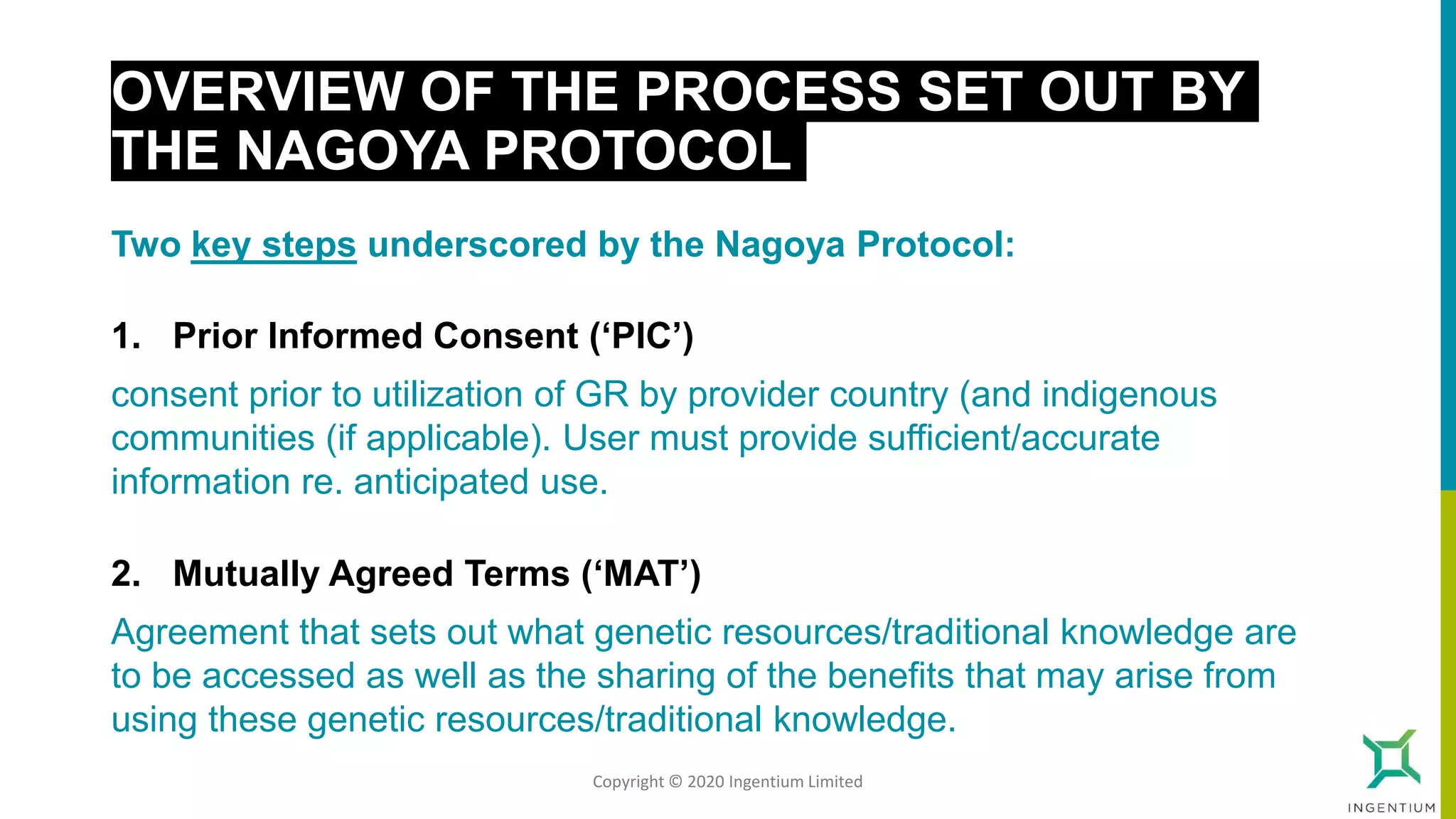
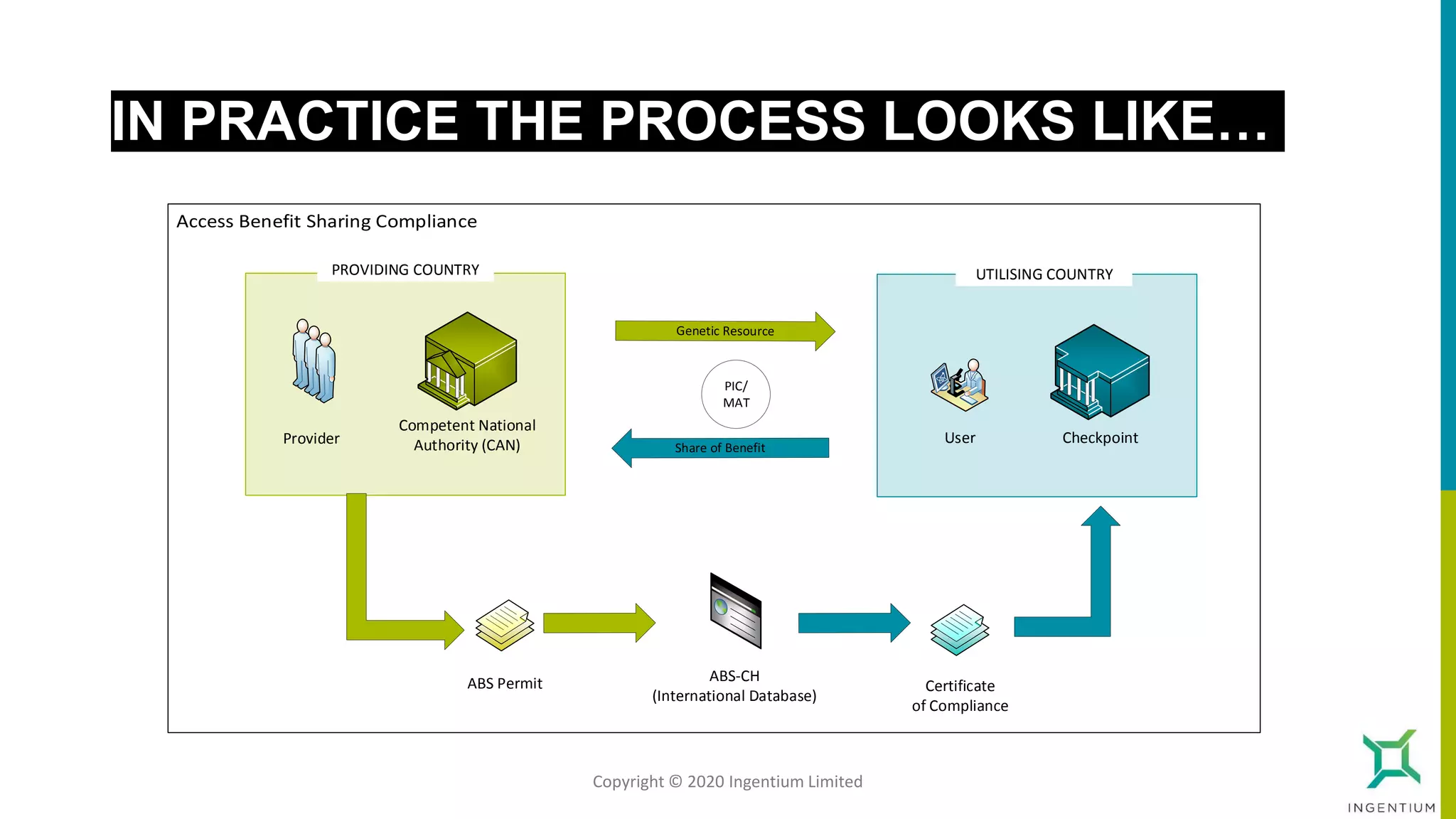
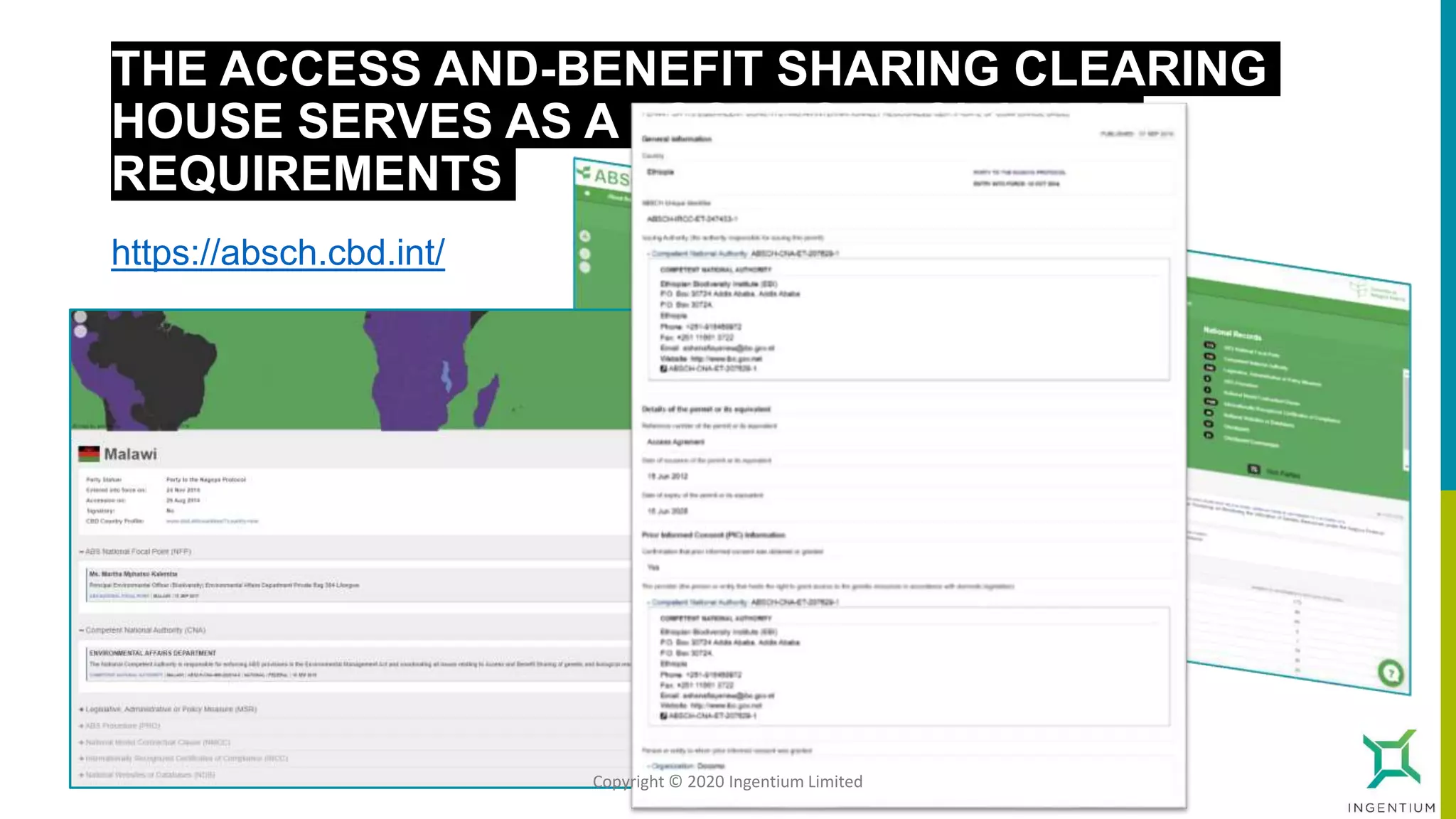
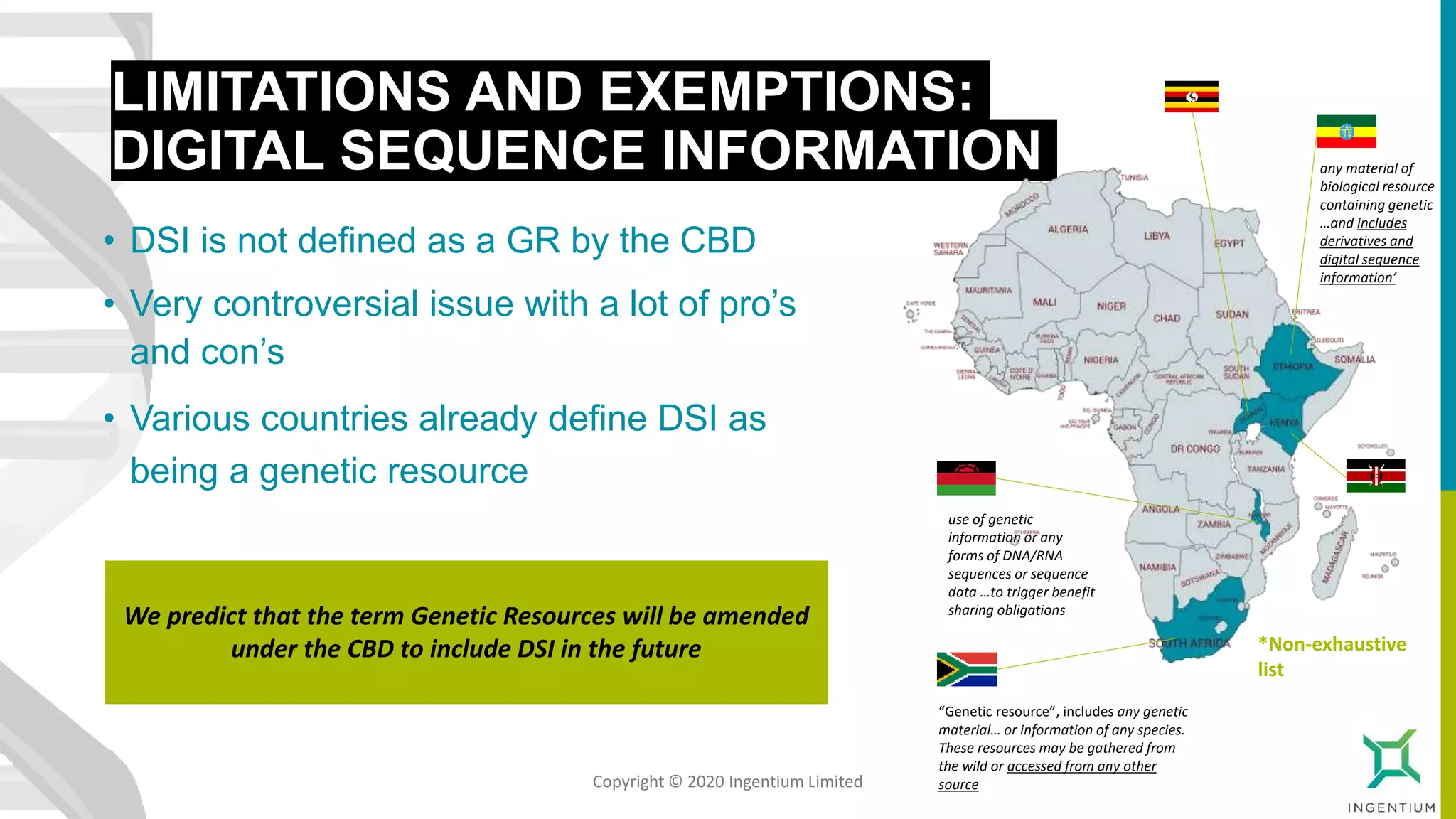
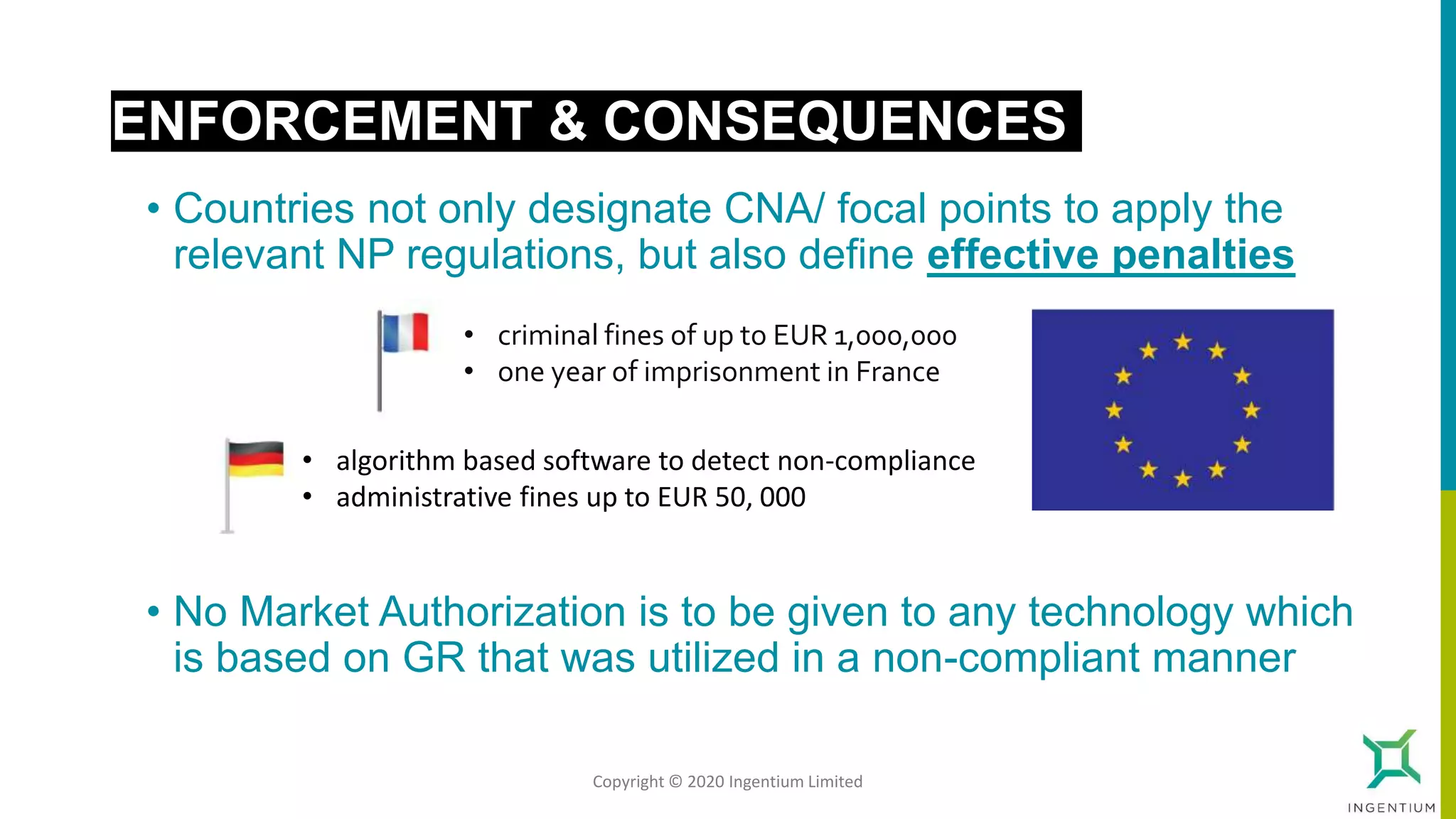
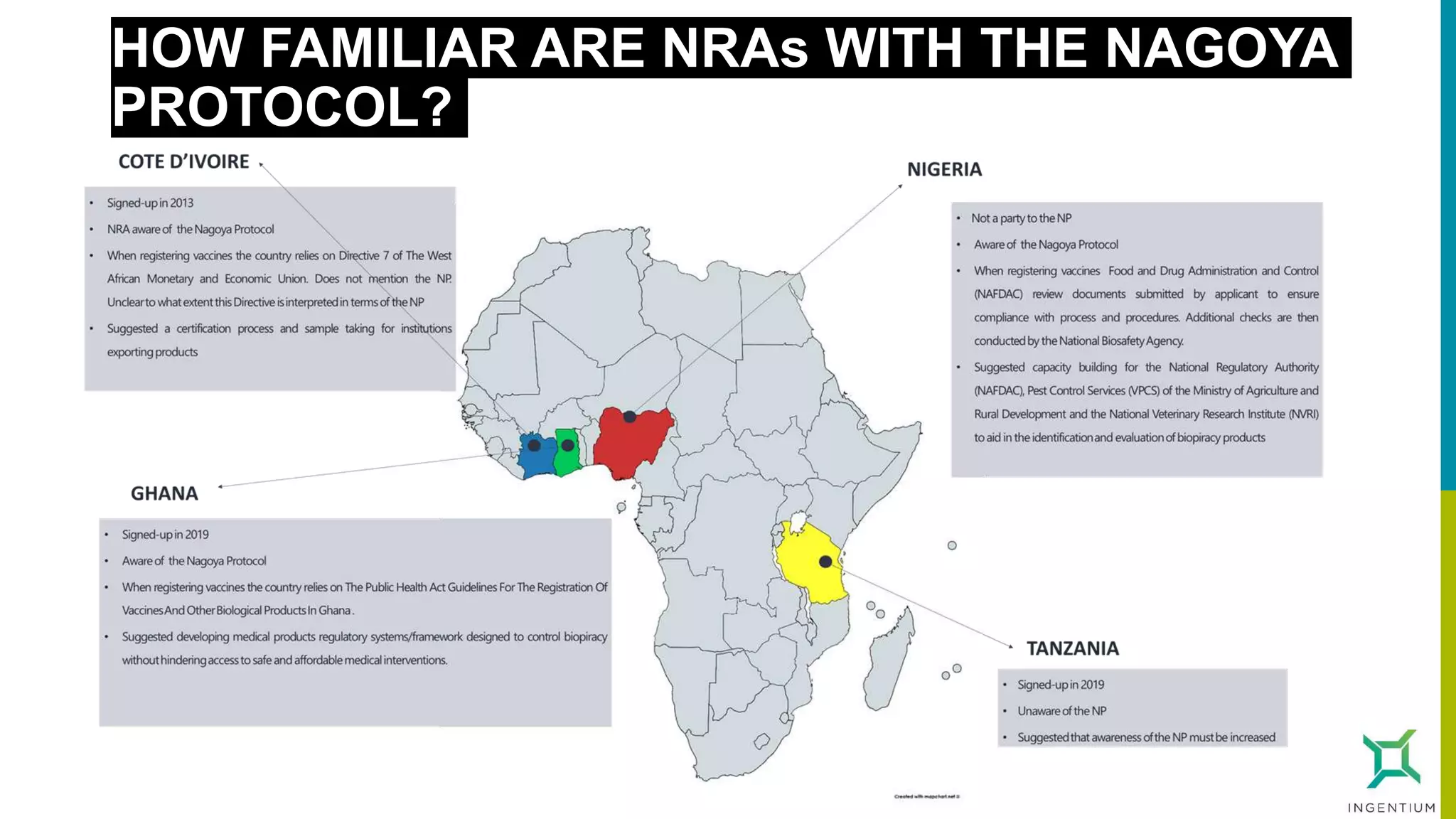
The document discusses the Nagoya Protocol, which is an extension of the Convention on Biological Diversity that establishes a framework for access to genetic resources and the sharing of benefits derived from their use. It outlines the requirements for prior informed consent and mutually agreed terms before utilizing genetic resources, along with exceptions for emergencies and the handling of digital sequence information. The document emphasizes the importance of compliance with these regulations and the potential penalties for non-compliance.