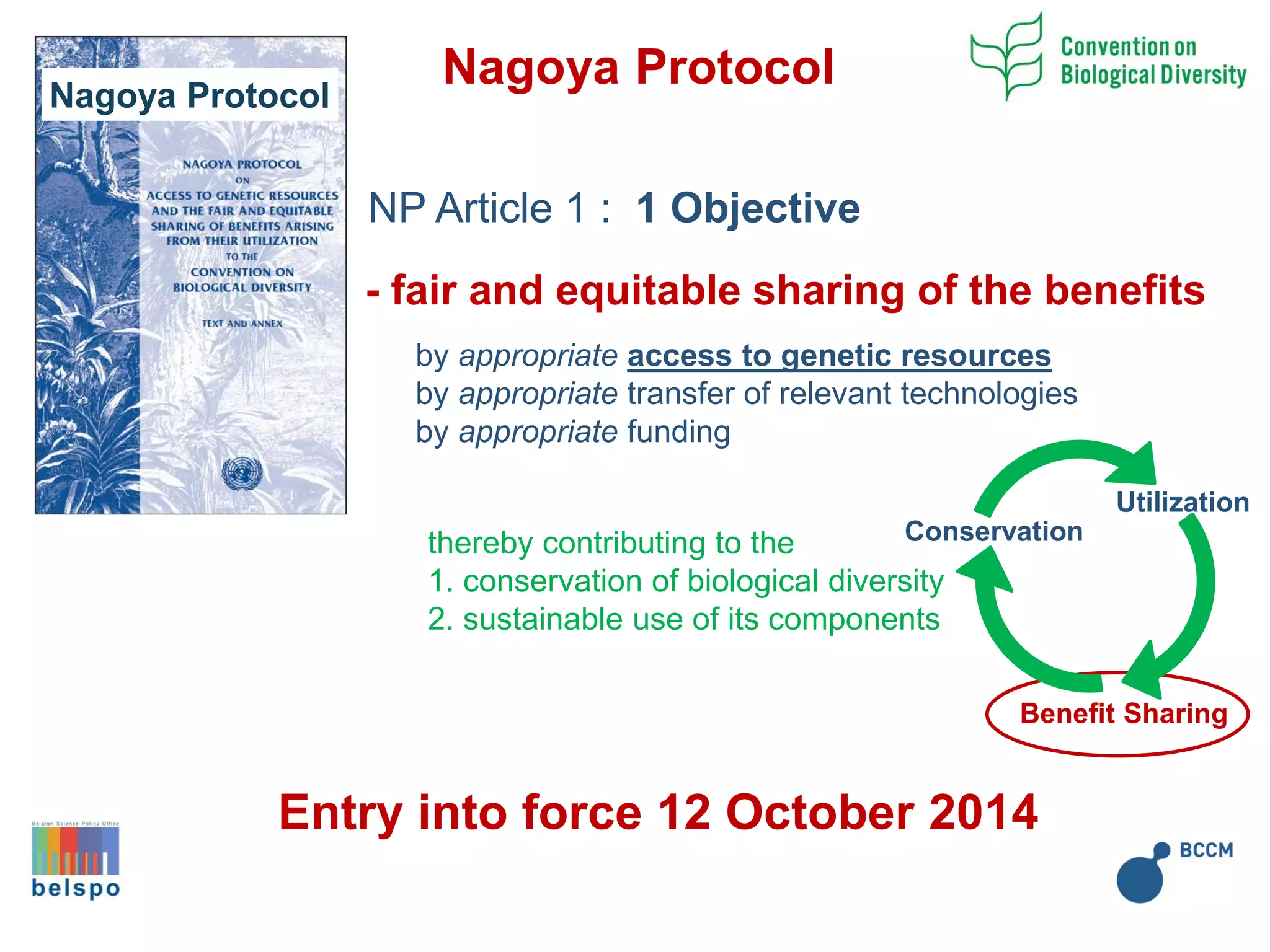
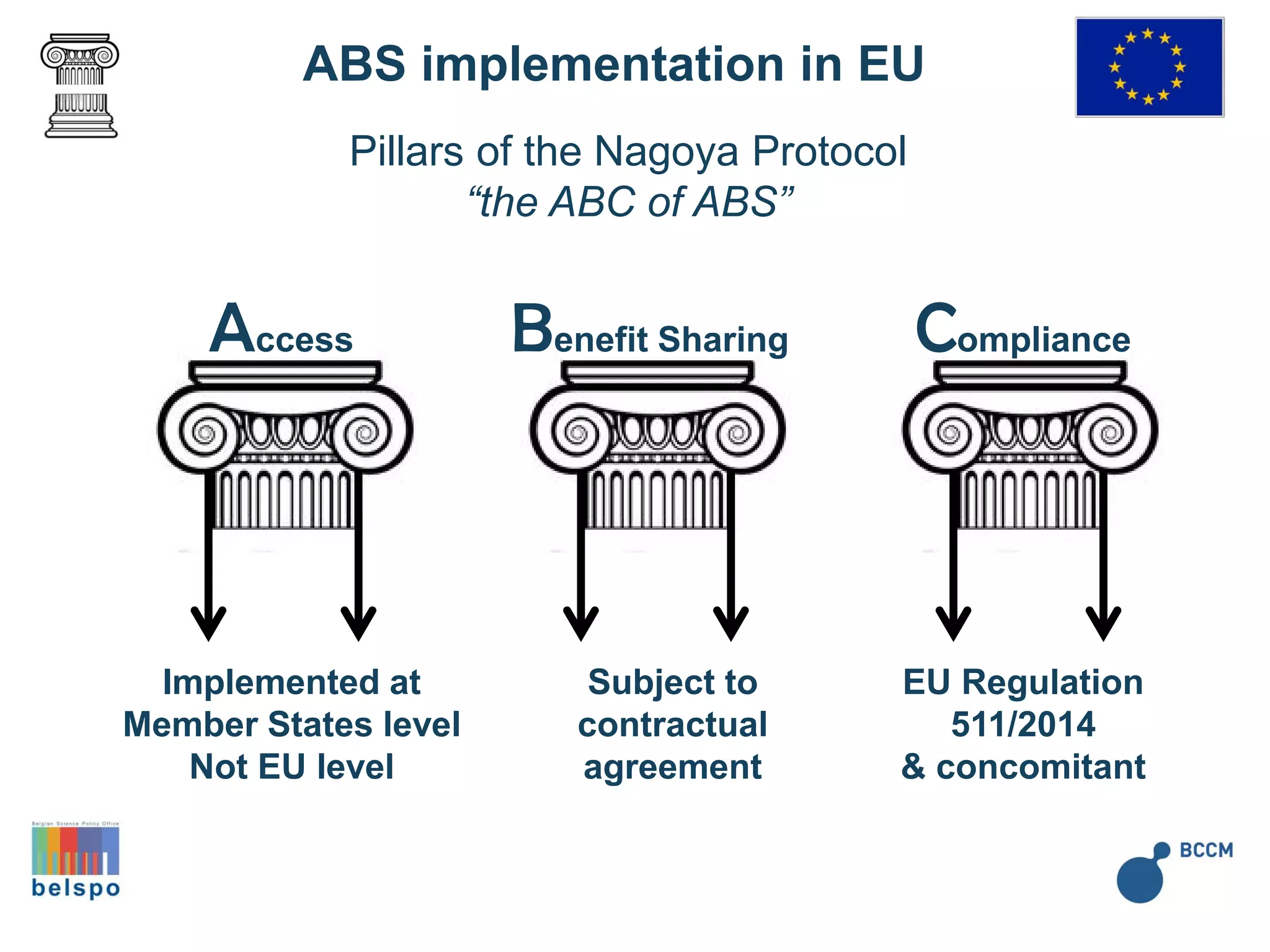
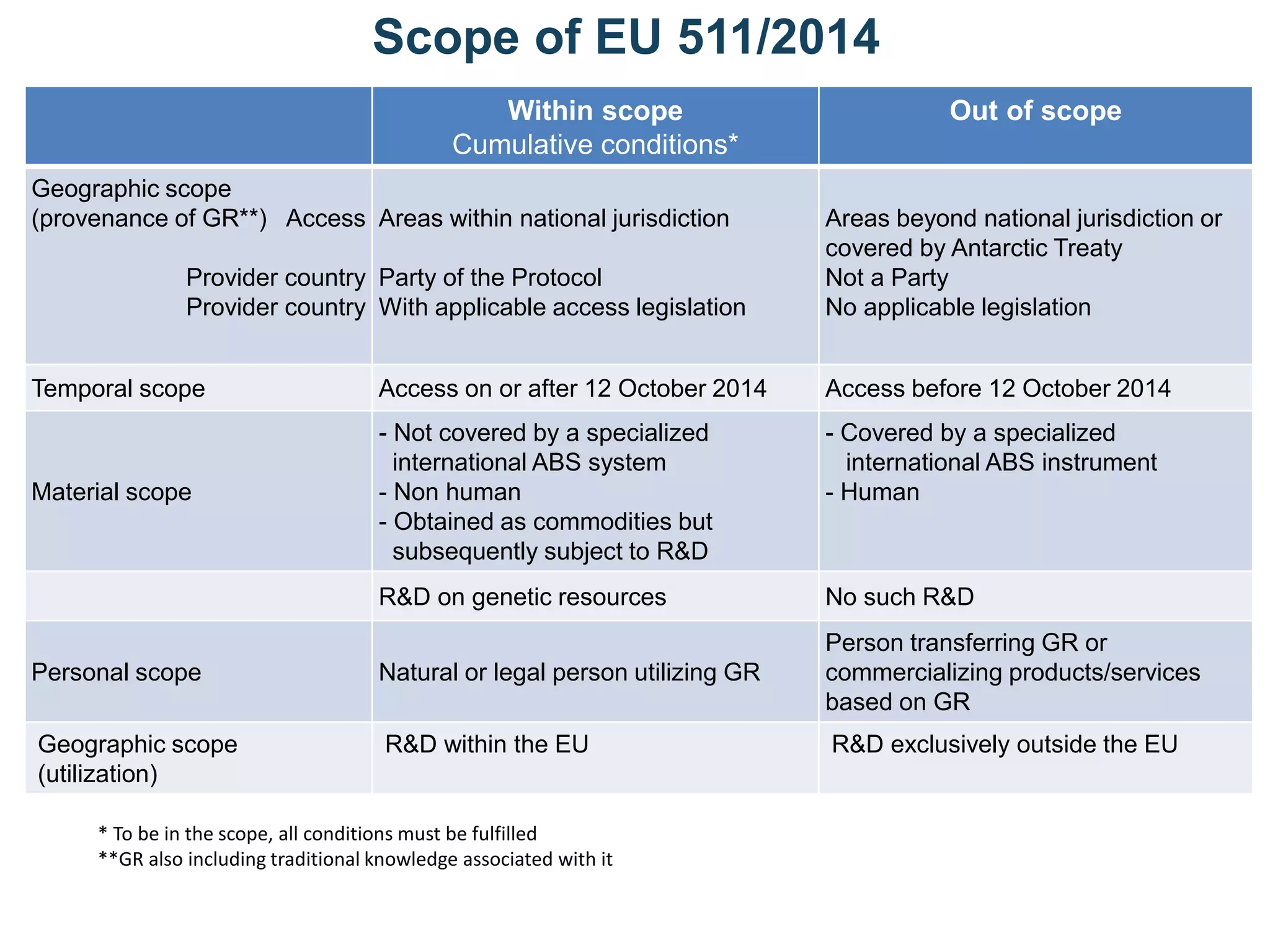
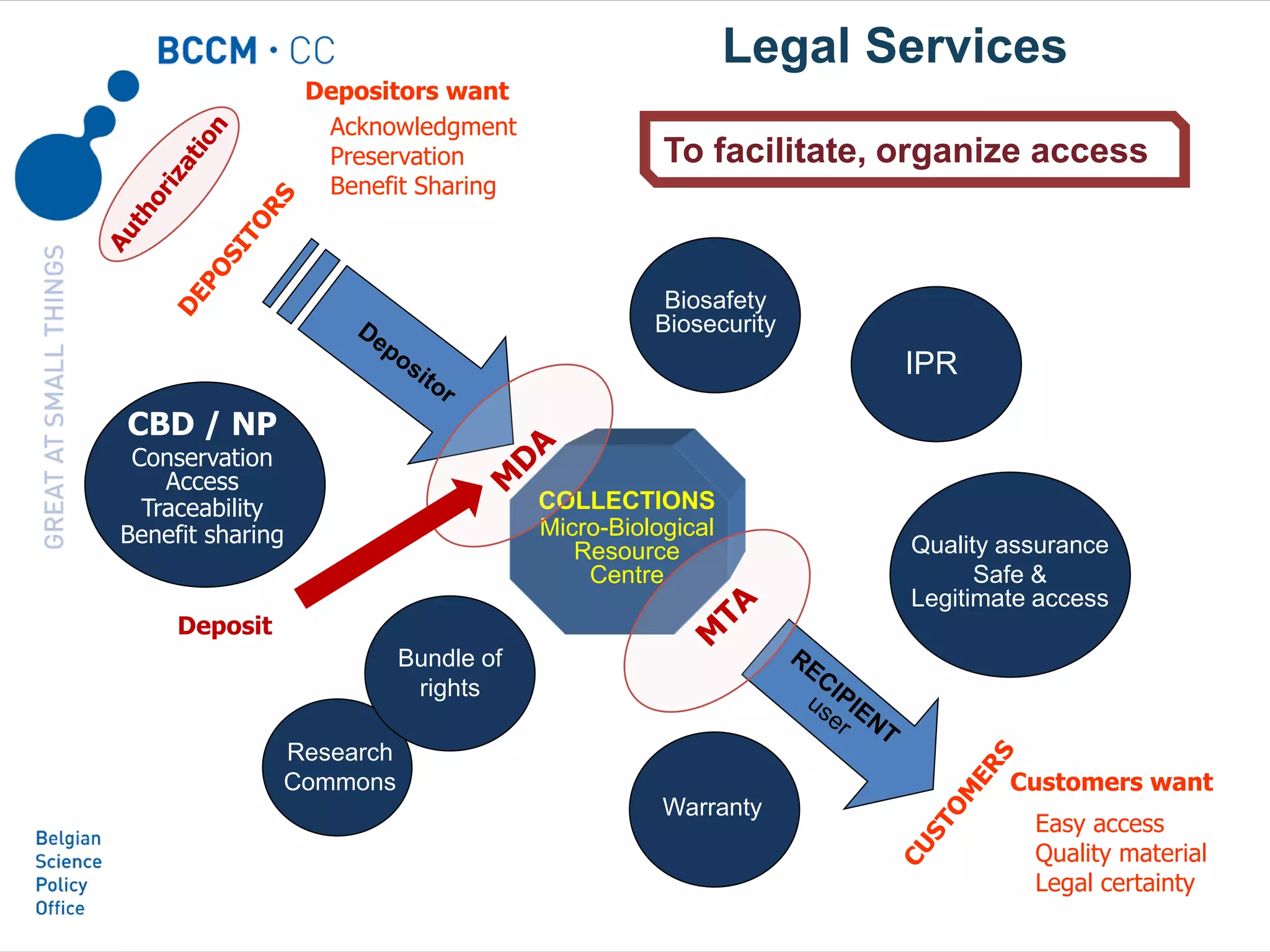
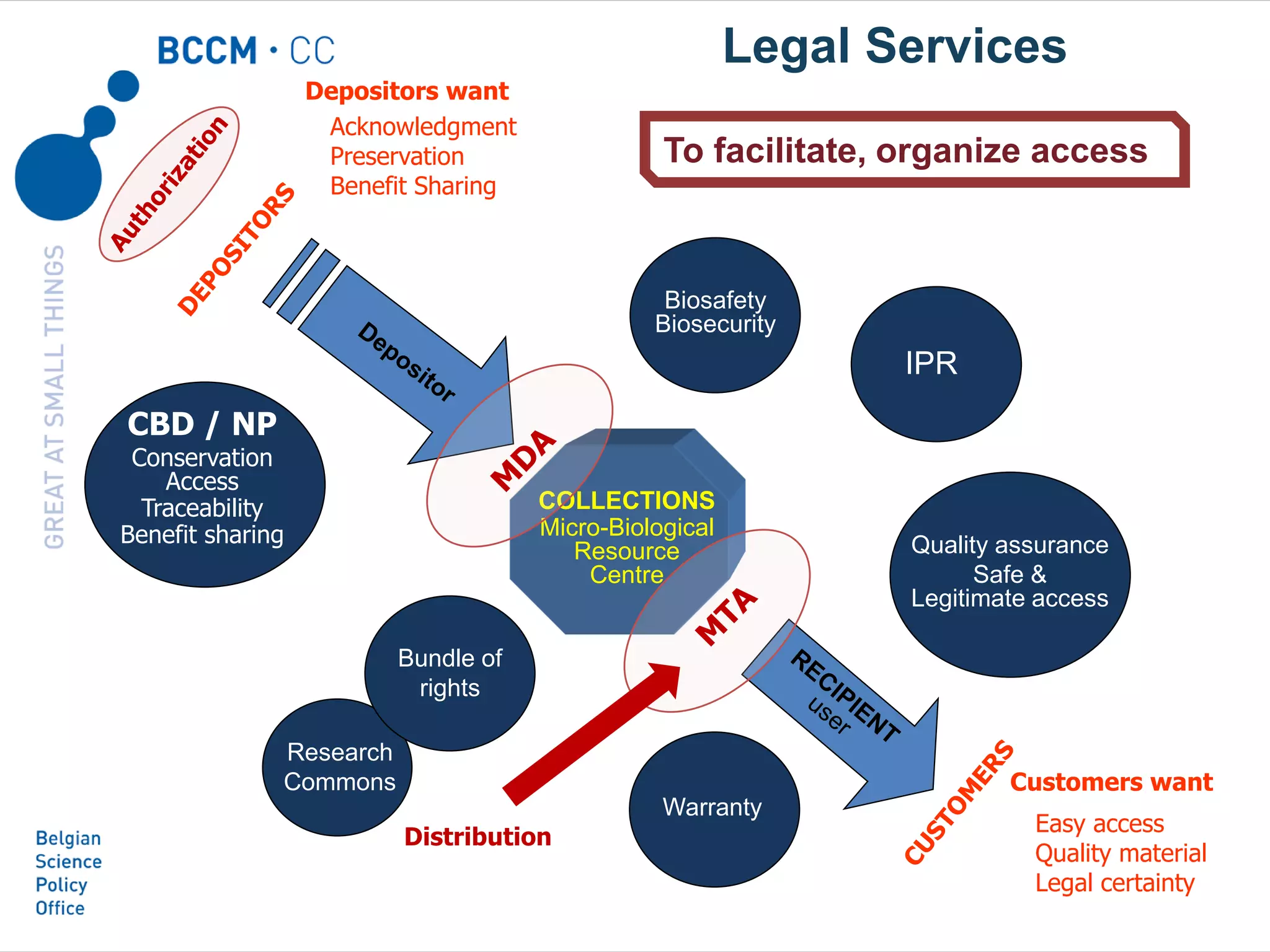
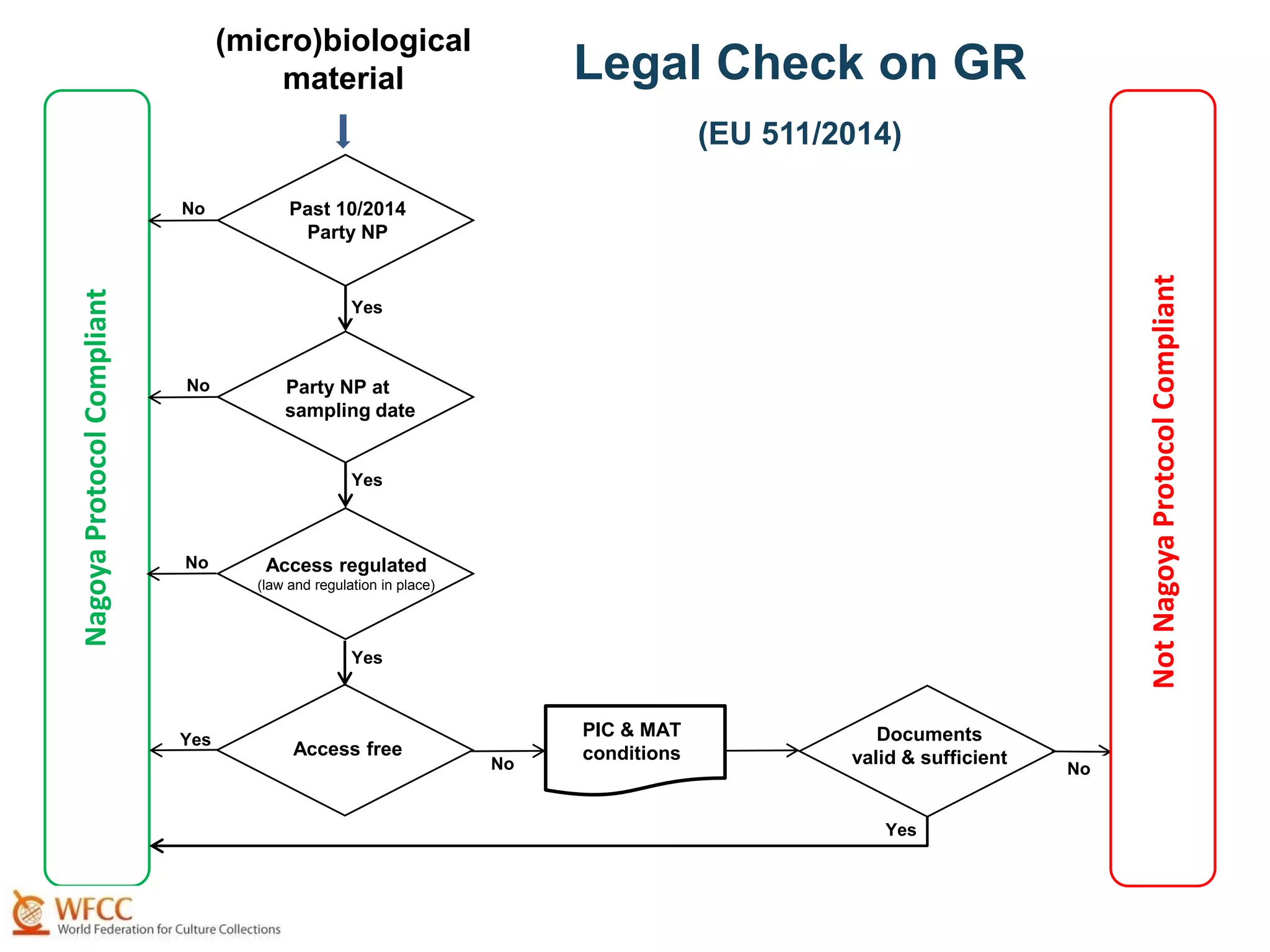
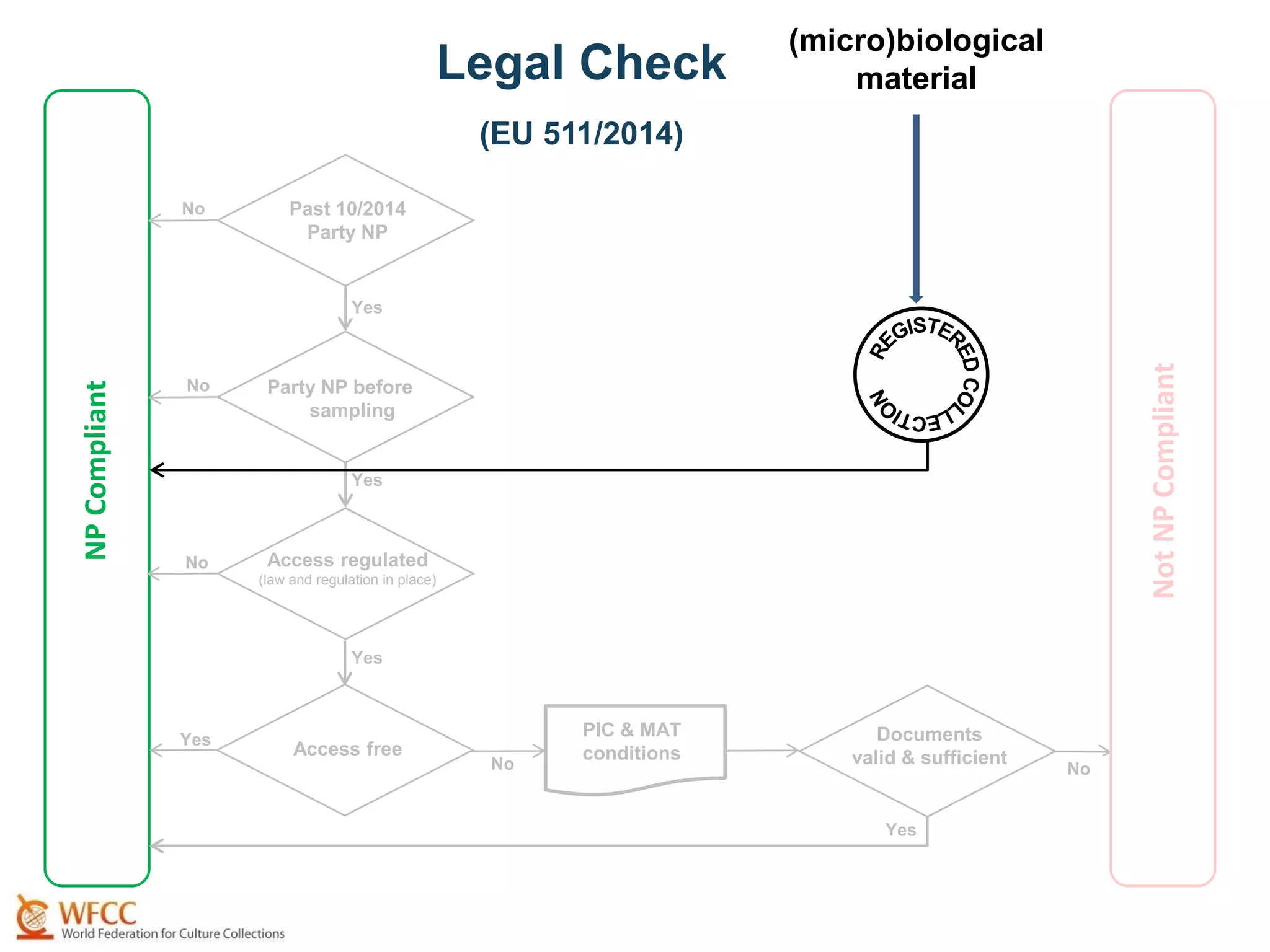
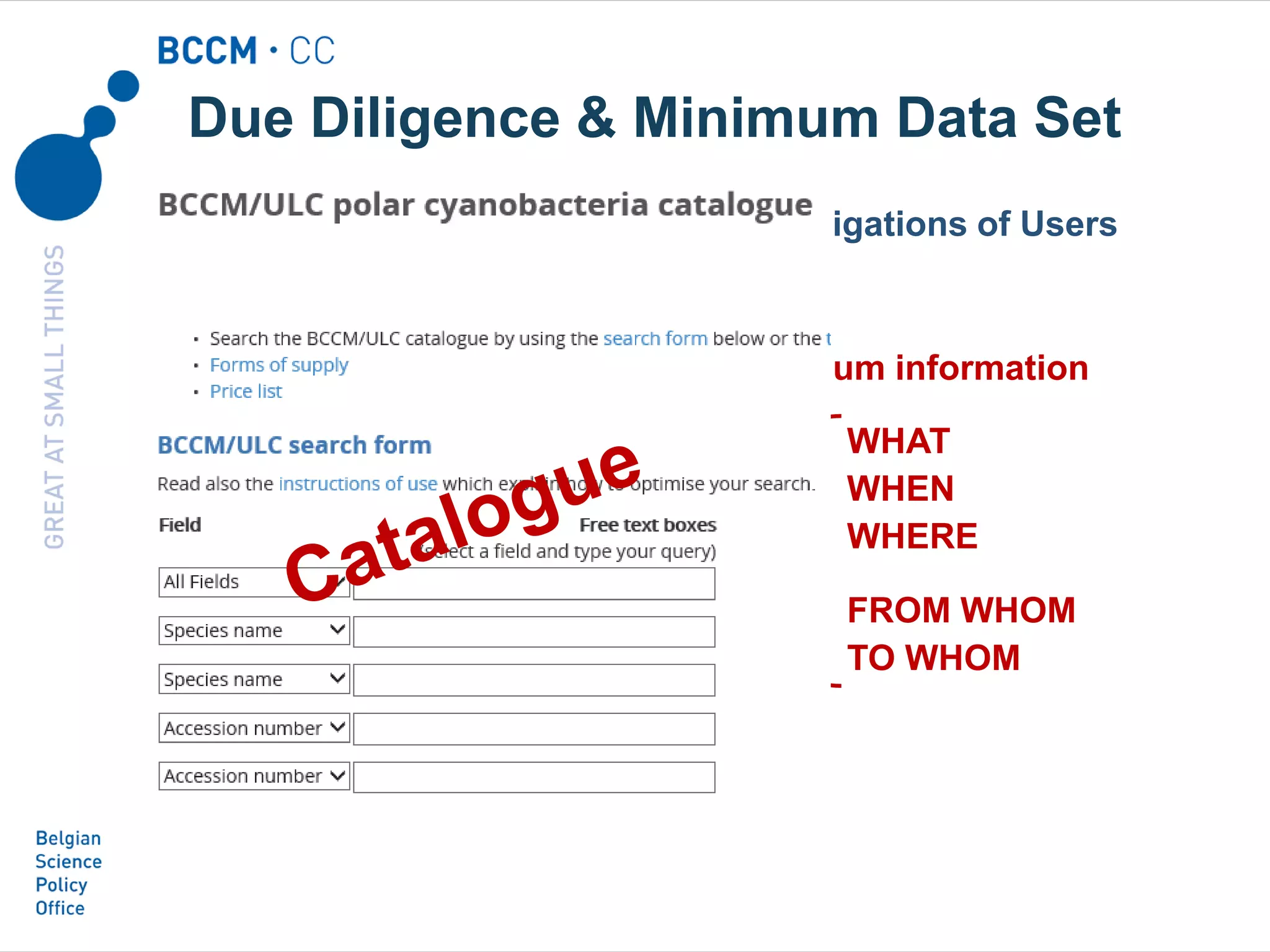
The document outlines the integration of the Nagoya Protocol's rules into corporate practices regarding genetic resources, emphasizing the importance of fair benefit sharing and sustainable use. It details the legal obligations and corporate policies necessary for compliance with access and benefit sharing regulations in research and development. Additionally, it provides guidance on obtaining prior informed consent and due diligence in the collection and utilization of biological resources.




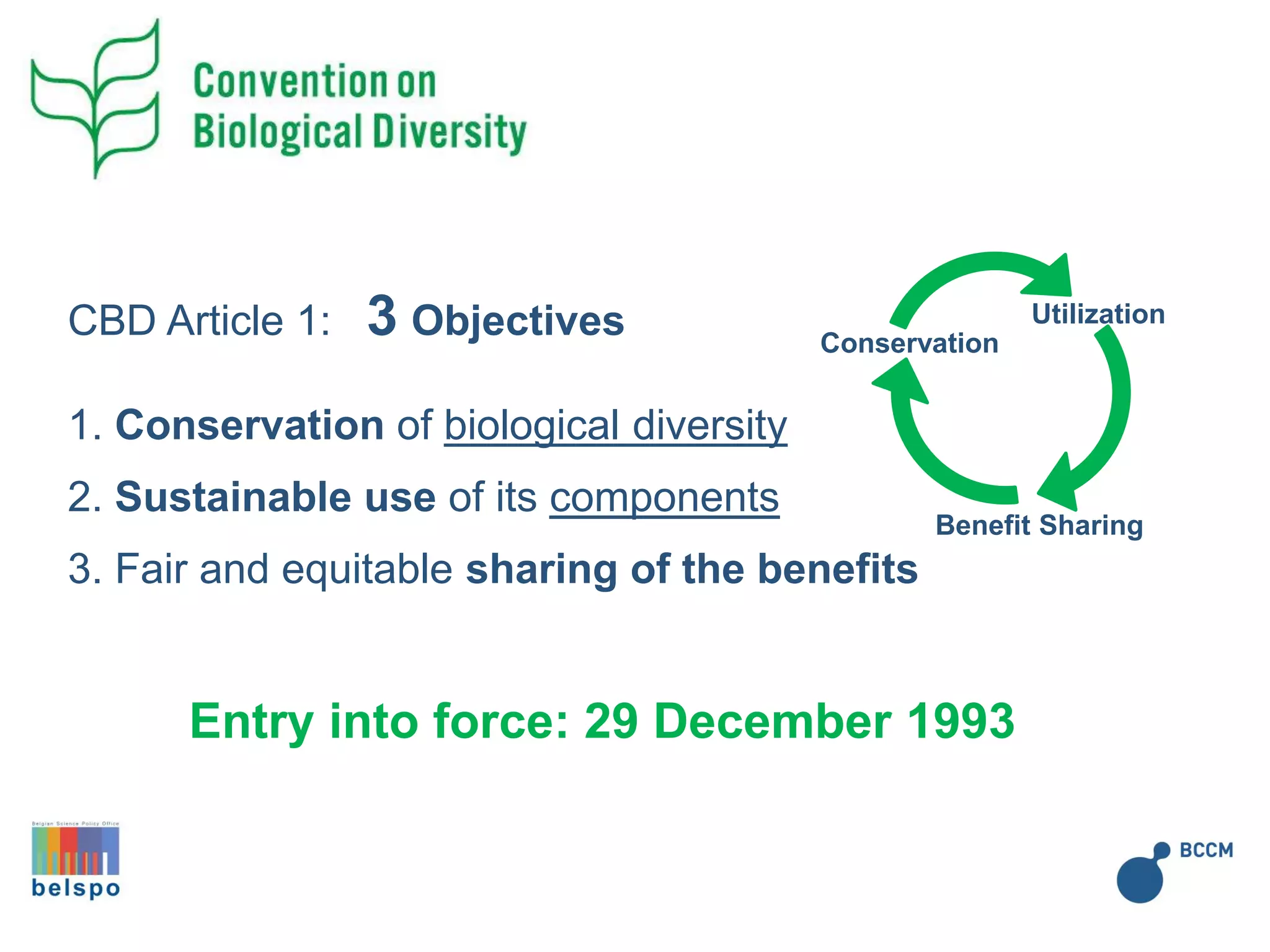
![From open access
[…the authority to determine access…] (CBD article 15.1)
[…to create conditions to facilitate access…] CBD art. (15.2)
From open access to sovereign rights (CBD article 15)
Change of paradigm](https://image.slidesharecdn.com/20171027protocolenagoyabelspo-171027133521/75/Protocole-NAGOYA-Le-Point-du-LIEGE-science-park-27-octobre-2017-6-2048.jpg)