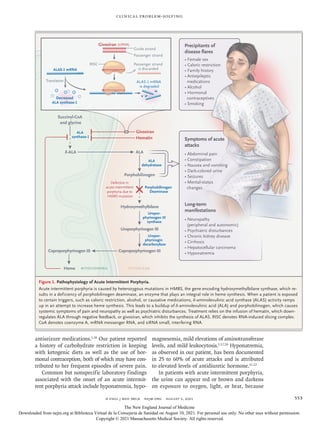
1) A 32-year-old woman presented with a 10-year history of severe recurrent abdominal pain.
2) Extensive prior testing and evaluations did not identify a cause, though she was diagnosed with conversion disorder during one hospitalization.
3) During her most recent hospitalization for abdominal pain, she developed hyponatremia that was initially thought to be due to poor oral intake but worsened despite IV fluids.
4) Further testing revealed findings consistent with SIADH and elevated urine porphyrin levels, leading to a diagnosis of acute intermittent porphyria, a rare genetic disorder causing episodic severe abdominal pain and other symptoms.