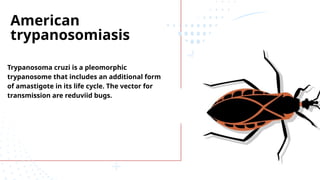
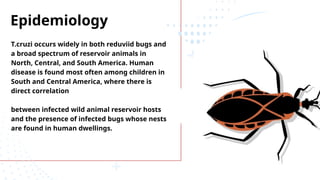
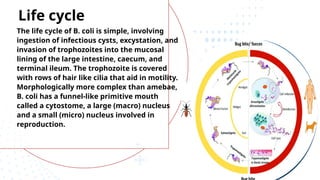
The document discusses various trypanosomiasis diseases caused by Trypanosoma species, including African trypanosomiasis (sleeping sickness) caused by Trypanosoma brucei and American trypanosomiasis (Chagas' disease) caused by Trypanosoma cruzi, detailing their life cycles, transmission, epidemiology, clinical features, and treatment options. It highlights the role of vectors such as tsetse flies and reduviid bugs in spreading these diseases, along with symptomatic manifestations and the importance of preventive measures. Additionally, the document briefly covers balantidiasis caused by Balantidium coli, its transmission, clinical aspects, and treatment protocols.