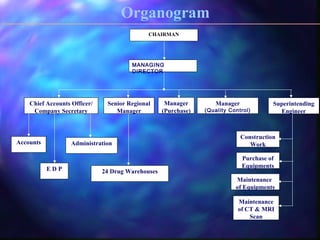
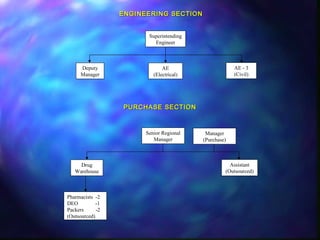
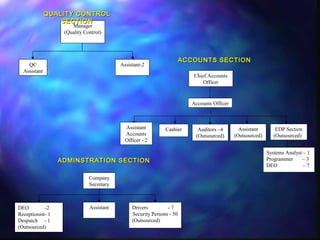
The document provides information about the Tamil Nadu Medical Services Corporation Limited (TNMSC), which supports government medical institutions in Tamil Nadu by procuring and distributing high quality drugs, medicines, surgical items and sutures. Some key details:
1) TNMSC was incorporated in 1994 and started operations in 1995, procuring drugs through a centralized tender process to ensure quality and competitive rates.
2) It serves over 11,000 government medical facilities across Tamil Nadu, including hospitals, primary health centers and veterinary institutions.
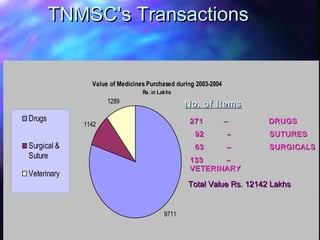
3) TNMSC's annual procurement is over Rs. 12,000 lakhs covering 271 drug items, 92 suture items and 63 surgical items. Rates have reduced