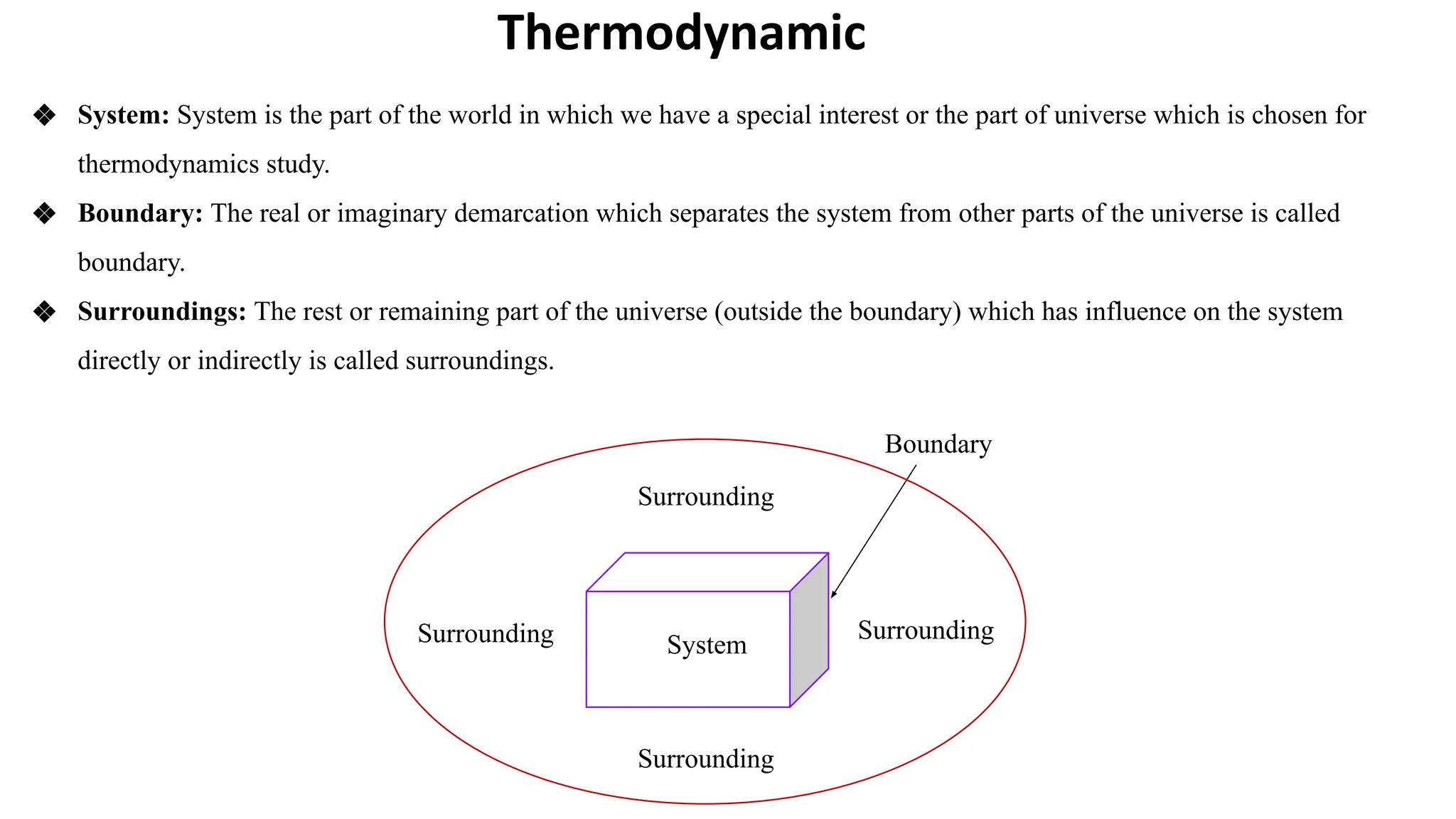
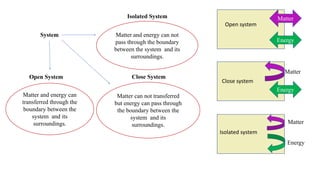
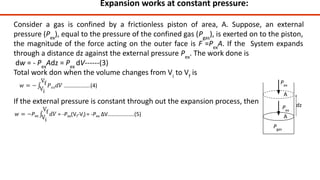
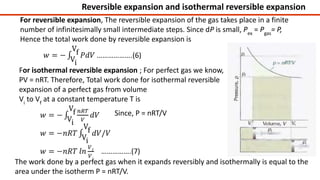
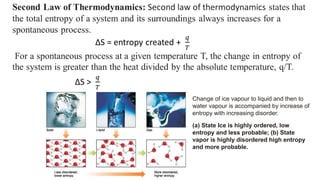
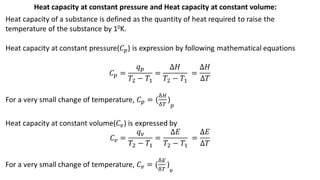
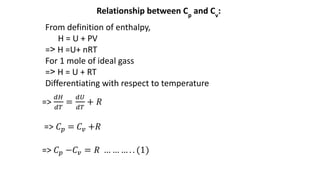
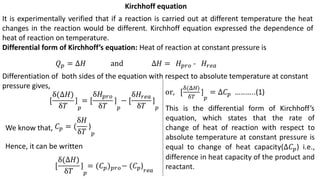
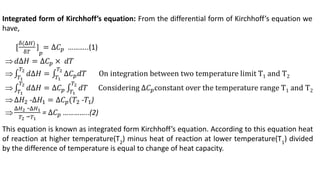
Thermodynamics is a branch of physics that deals with the relationships between heat, work, temperature, and energy. It describes how thermal energy is converted to and from other forms of energy and how matter is affected by these processes. Thermodynamics is fundamental in science and engineering, especially in fields such as mechanical engineering, physical chemistry, and energy systems.